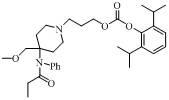
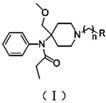
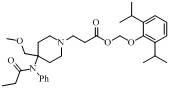
4-methoxy methyl-4-(N-propionyl) aniline piperidine compound and preparation method and application thereof
A technology of methoxymethyl and aniline piperidine, applied in the field of 4-methoxymethyl-4-aniline piperidine compounds, which can solve problems such as slow recovery, waste of medical resources, and increased operational complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve the compound of formula (Ⅳ) (CAS number: 61086-18-8) (65 mg, 0.236 mmol) and acrylic acid (51 mg, 0.708 mmol) in 20ml di Chloromethane, stirred overnight at room temperature. After the reaction solution was concentrated, it was purified by preparative thin layer (dichloromethane / methanol=8:1) to obtain 56 mg of a yellow oily intermediate, with a yield of 68%.
[0018] .
[0019] This intermediate (56 mg, 0.161 mmol), Cs 2 CO 3 (105 mg, 0.322 mmol) and (55 mg, 0.241 mmol) were dissolved in 10ml of acetone and stirred overnight at room temperature. After the reaction solution was concentrated, it was purified by thin layer (developing solvent: cyclohexane / ethyl acetate = 1:1), and n=2 and R in the formula (I) were 30 mg of yellow oily product, yield 34%.
[0020] Product structure detection results:
[0021] NMR instrument: Bruker WH-300 (300 MHz) spectrometer, TMS is the internal standard, and the unit of δ is ppm.
[0022] Mass spectrometer: Agilent ...
Embodiment 2
[0026] The compound of formula (IV) (138 mg, 0.5 mmol), triethylamine (253 mg, 2.5 mmol) (or pyridine 200 mg, 2.5 mmol) and 3-bromo-n-propanol ( 348 mg, 2.5 mmol) was dissolved in 4ml of dichloromethane, stirred overnight at room temperature. After the reaction solution was concentrated and purified by thin layer (developing solvent: dichloromethane / methanol = 6:1), 170 mg of a yellow oily intermediate was obtained, with a yield of 99% (when the acid removal agent was pyridine, 160 mg of the intermediate was obtained, with a yield of 93%).
[0027] After propofol chloroformate was prepared from propofol and phosgene according to the reported method, the above-mentioned intermediate (170 mg, 0.5 mmol), triethylamine (101 mg, 1 mmol) and propofol Porphenol chloroformate was dissolved in 20ml of dichloromethane, stirred overnight at room temperature, and the reaction solution was concentrated and purified by preparative thin layer (developing solvent: cyclohexane / ethyl acetate =...
Embodiment 3
[0034] Pharmacological activity experiment:
[0035] 1. Anesthetic activity test:
[0036] 20 male Kunming mice with a body weight of 20-30 grams were randomly divided into 4 groups, and the compound of the present invention was prepared into a solution with normal saline, and 5 mice in each group were administered via the tail vein, and the disappearance of the righting reaction was observed. No, as an indicator of narcotic effects. The results of the anesthetic activity experiments are shown in Table 1.
[0037] Table 1 The anesthetic activity test result of the compound of the present invention
[0038] compound Dose (mg / kg) Is the righting reflex gone? normal saline — no Propofol 15 yes
[0039] The results in Table 1 show that the compound of the present invention has an anesthetic effect and can cause the reversible disappearance of righting reflex in mice.
[0040] 2. Analgesic activity test:
[0041]At room temperature of 20°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com