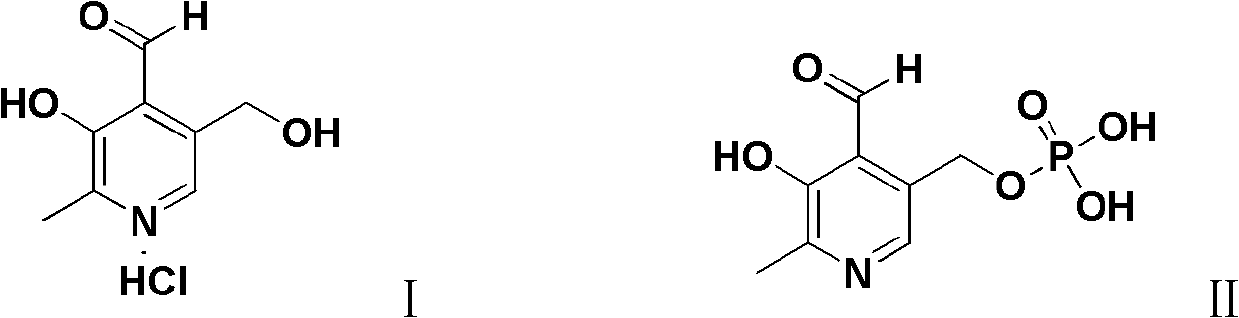
Preparation method of pyridoxal or pyridoxal hydrochloride
A technology for pyridoxal hydrochloride and pyridoxal hydrochloride, which is applied in the field of preparation of pyridoxal or pyridoxal hydrochloride, can solve the problems of large reaction pollution and complicated process, and achieves good selectivity, high conversion rate and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a 25mL reaction flask with a stirrer and a thermometer, add 0.085g (0.5mmol) 2,2,6,6-tetramethylpiperidine-nitrogen-oxygen radical, 0.29g (2mmol) copper bromide successively, 0.40g (5mmol) pyridine and 2.06g (10mmol) pyridoxine hydrochloride. Add 5 mL of water, control the temperature at 30°C, and start stirring. Then 2.0 g (18 mmol) of 30% hydrogen peroxide was added dropwise while controlling the reaction temperature at 30-35° C. After the dropwise addition, keep stirring at 30-35° C. for 1-2 hours, and the obtained reaction solution detects that the pyridoxine conversion rate reaches 99% (liquid phase conditions: the chromatographic column is XDB-G8 250×4.6mm 5um. The mobile phase is methanol: buffer=15:85; buffer: 0.04% sodium pentanesulfonate and adjust the pH to 3 with glacial acetic acid; the detection wavelength is 284nm), and the reaction selectivity is 98%.
[0031] In addition, take about 5mL of the reaction solution and put it directly on the silica gel...
Embodiment 2
[0034] The oxygen source was changed to 1 atmosphere of oxygen, the inorganic salt was changed to 1.5g (10mmol) copper nitrate, the amine ligand was changed to 1.0g (10mmol) triethylamine, and other operations were the same as in Example 1 to obtain 2.21g of a yellow solid. The conversion rate of pyridoxine is up to 99% and the reaction selectivity is 97% according to liquid phase detection. The overall yield of the two-step reaction was 89%.
Embodiment 3
[0036] Oxygen source is changed into the oxygen of 2 atmospheric pressures, and inorganic salt is changed into 0.29g (2mmol) cuprous bromide, and amine ligand is changed into 0.24g (4mmol) 1,2-ethylenediamine, and other operation is with embodiment 1, 1.90 g of a yellow solid was obtained. The conversion rate of pyridoxine is up to 87% and the reaction selectivity is 95% according to liquid phase detection. The overall yield of the two-step reaction was 74%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com