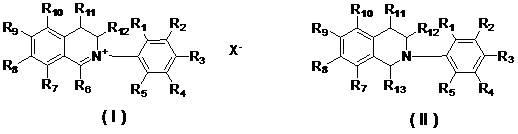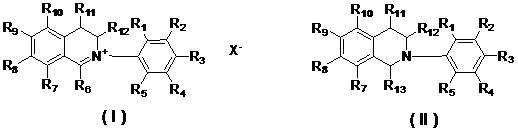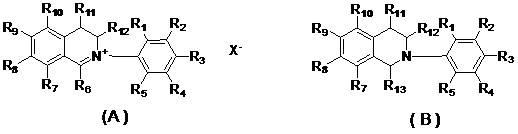Two types of isoquinoline compounds and application thereof to preparing anti-cancer medicaments
An anti-cancer drug, the technology of isoquinoline, is applied in the direction of boron compound active ingredient, compound containing periodic table Group 3/13 elements, anti-tumor drug, etc. Research reports on cancer activity, literature reports on unseen structures and synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] In the present invention, applicants designed and synthesized N-aryl-3,4-dihydroisoquinolinate (A series) and 1-cyano or alkoxy-N-aryl-1,2,3 , The general structural formula of 4-tetrahydroisoquinoline compound (B series) is as follows:
[0015]
[0016] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 are the same or different hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, unsaturated monocyclic hydrocarbon, alkoxy, halogen, hydroxyl, nitro, cyano, trifluoromethyl, heterocycle substituent, carboxyl, ester, amide, acyl or aldehyde;
[0017] R 6 is an aliphatic or aryl group;
[0018] R 13 Is cyano (—CN) or alkoxyl (RO—);
[0019] x - Sulfate, halide anion, carbonate, bicarbonate, phosphate, hydrogen phosphate, fatty acid, sulfonate or tetraphenylborate.
[0020] The invention established for the preparation of N-aryl-3,4-dihydroisoquinolinate (A series) and 1-cyano or alkoxy-N-aryl-1,2,3,4-tetra The typical synthetic ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com