Polymerase chain reaction (PCR) detection method universal for viruses
A detection method and virus technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of low repeatability, high quality requirements of virus samples, and not well promoted, and achieve the solution Cumbersome, specific and reproducible effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
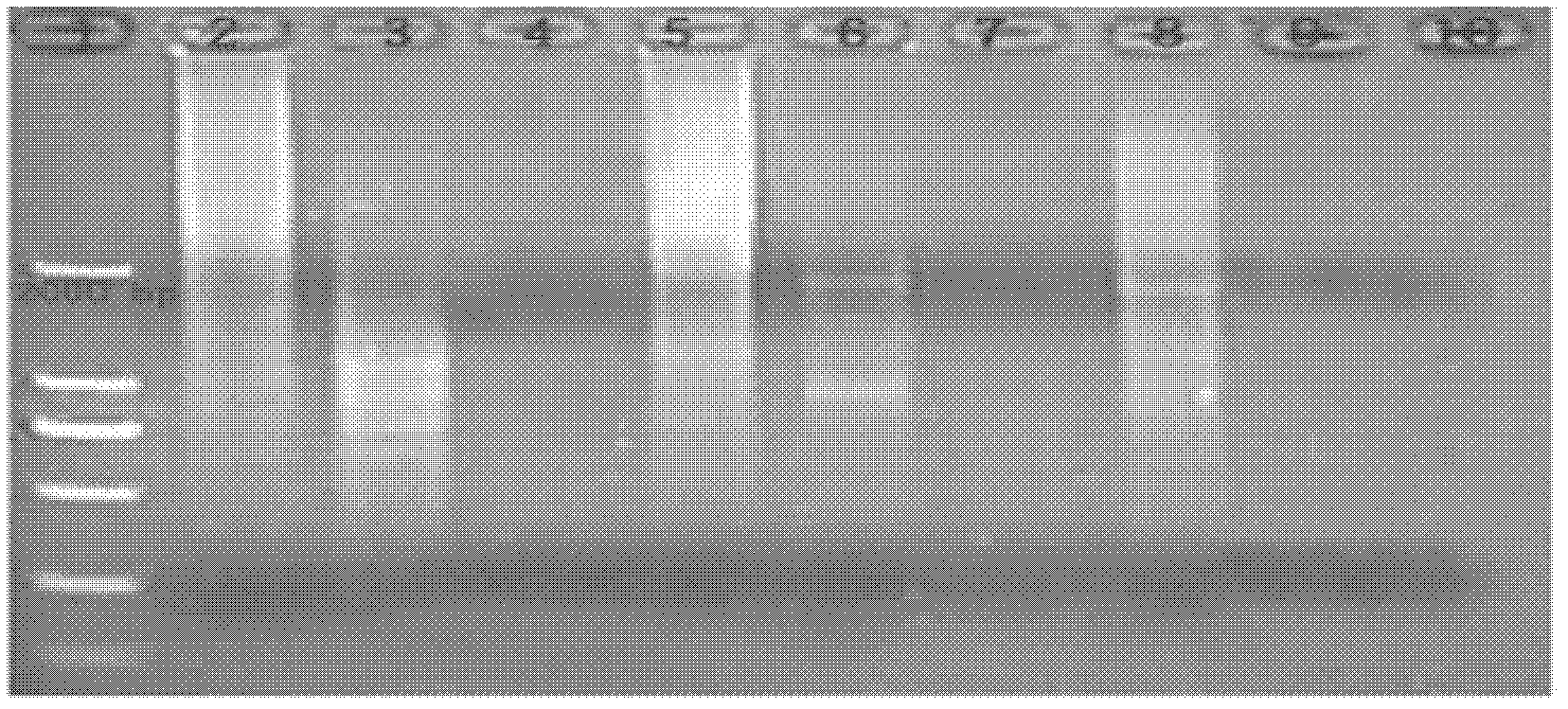
[0044] Embodiment 1: virus general PCR method specificity test
[0045] 5. Materials
[0046] Virus strains and cells: Specificity test to detect viruses (as shown in the table below) and strains are preserved by the crude drug research and development center of Guangdong Dahuanong Animal Health Products Co., Ltd.
[0047]
[0048] Strain: DH5α strain is preserved by the crude drug research and development center of Guangdong Dahuanong Animal Health Products Co., Ltd.
[0049] Random primer: VVVVVVVVAA, where V is any one of T, G, and C, synthesized by TaKaRa Company; after sequencing, the random primers corresponding to each virus are: H9N1: TTGGGGCGAA;
[0050] PRRSV: GCTGCCTTAA / CGTCTGCTAA; PRV: CTGCTGCTAA;
[0051] IBV: TTGGTGGCAA; EDS: TGTTTCTCAA; NDV: TTTCTCGGAA; PRRSV-PRV: GGCGTGTGAA / CTTGGGGCAA.
[0052] 2. Method steps
[0053] Virus propagation and culture are shown in the following table:
[0054]
[0055] Cell venom sample pretreatment: the above-mentioned...
Embodiment 2
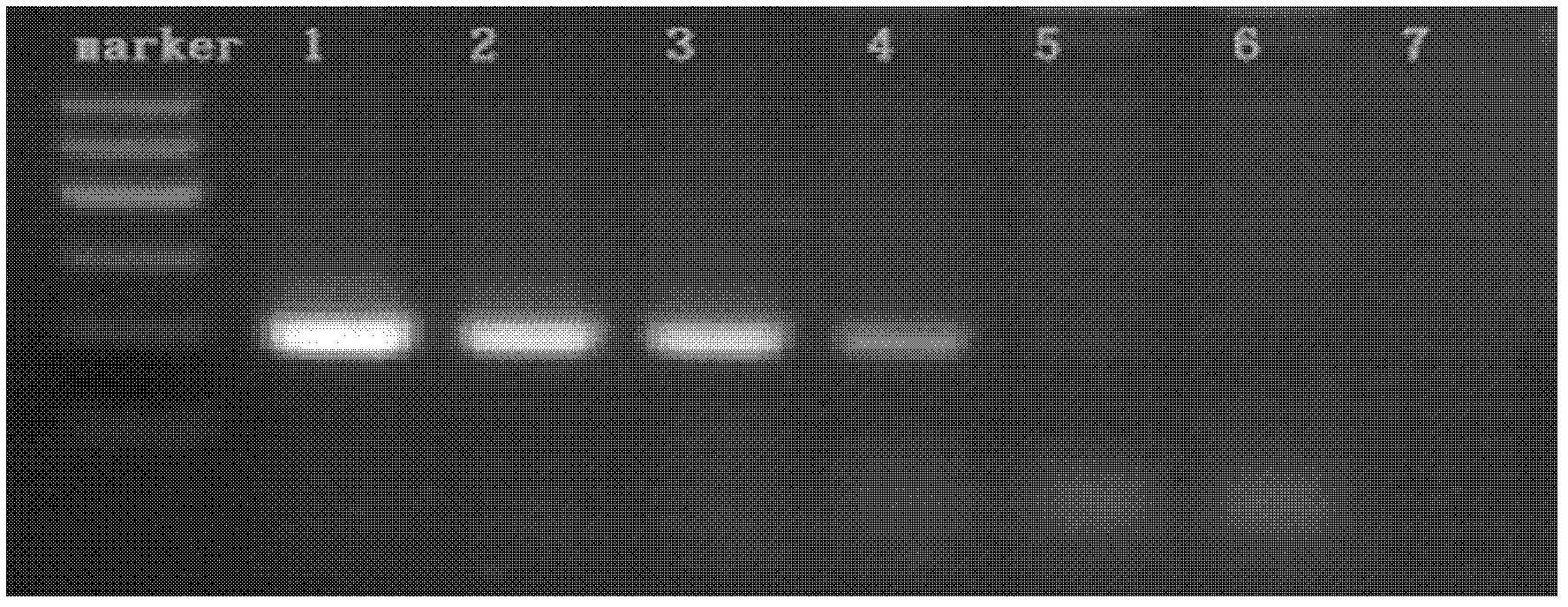
[0068] Embodiment 2: virus universal PCR method susceptibility test
[0069] 5. Materials
[0070] Virus strain, cell: PRV recombinant virus PRV / TK with TK gene deletion of PRV-Ra strain - : Constructed and preserved by the crude drug research and development center of Guangdong Dahuanong Animal Health Products Co., Ltd.; BHK21 cells were purchased from China Veterinary Drug Control Institute.
[0071] Strain: DH5α strain is preserved by the crude drug research and development center of Guangdong Dahuanong Animal Health Products Co., Ltd.
[0072] Random primer: VVVVVVVVAA, where V is any one of T, G, and C, synthesized by TaKaRa Company.
[0073] 2. Method steps
[0074] Virus proliferation: BHK21 cells were cultured according to conventional methods, infected with virus at 5-10 PFU / cell, when the cytopathic pathology (CPE) reached about 90%, the virus was harvested, and the TCID50 of the virus was calculated;
[0075] Cell venom sample pretreatment: take 107.0TCID50 cell...
Embodiment 3
[0087] Example 3: Identification of Unknown Microorganisms
[0088] In a chicken farm, chicken embryos were inoculated with the liver suspension of sick and dead chickens. The whole body of the chicken embryos was severely hemorrhage, and blister-like substances appeared on the allantoic membrane. Various methods such as PCR and serology could not identify unknown microorganisms in the allantoic fluid; this test was carried out to identify the unknown microorganism.
[0089] 2. Materials
[0090] Chicken embryos: purchased from Guangdong Dahuanong
[0091] Random primer: VVVVVVVVAA, where V is any one of T, G, and C, synthesized by TaKaRa Company.
[0092] 3. Method steps
[0093] 1) Microbial proliferation: chicken embryo allantoic cavity inoculation: chicken embryo allantoic fluid containing unknown microorganisms was filtered through a 0.1um filter, 10-day-old SPF chicken embryo allantoic cavity was inoculated, observed for 3-5 days, and chicken embryo allantoic fluid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com