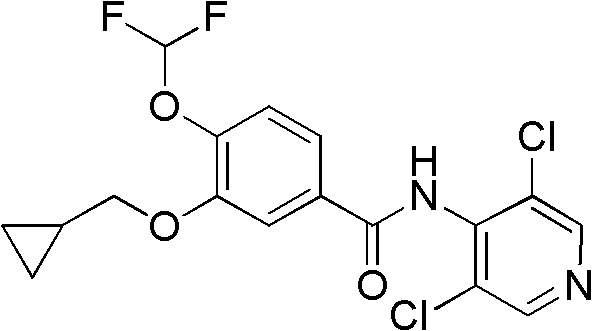
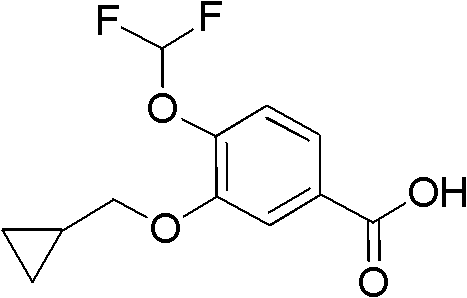
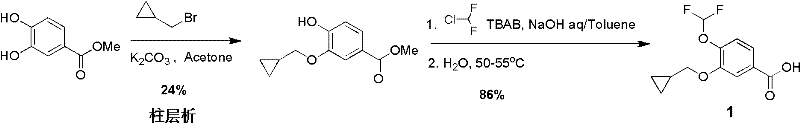
Method for preparing 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid
A technology of difluoromethoxybenzoic acid and cyclopropylmethoxy, which is applied in the field of preparation of the chronic obstructive pulmonary disease drug roflumilast, can solve problems such as difficult industrial scale-up production, and achieve simple and large-scale production. The effect of practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Methyl 3-cyclopropylmethoxy-4-iodobenzoate
[0075] 27.8 g of methyl 3-hydroxy-4-iodobenzoate, 27.6 g of potassium carbonate, 20.3 g of bromomethylcyclopropane, and 150 mL of DMF were stirred at 70° C. for 3 hours. After the reaction was diluted with 450 g of water, it was extracted with ethyl acetate, washed with water, and the organic layer was separated, washed with water, and dried. The solvent was distilled off under reduced pressure to obtain 32.9 g of methyl 3-cyclopropylmethoxy-4-iodobenzoate with a yield of 99%.
[0076] 1 H NMR (CDCl 3 ): δ0.42-0.48(m, 2H), 0.64-0.71(m, 2H), 1.25-1.45(m, 1H), 3.93(s, 3H), 3.98(d, 2H), 7.38(d, 1H ), 7.42(s, 1H), 7.87(d, 1H).
Embodiment 2
[0078] 3-Cyclopropylmethoxy-4-hydroxybenzoic acid (4)
[0079] Add 1.9 g of cuprous iodide, 2.9 g of 8-hydroxypyridine, 100 mL of DMSO and 32.9 g of methyl 3-cyclopropylmethoxy-4-iodobenzoate obtained in Example 1 into the reactor. With further stirring, 100 mL of 25% potassium hydroxide aqueous solution was added. Heated to 100°C for 24 hours, cooled to room temperature and filtered to remove the copper catalyst. Adjust to acidity with hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 19.6 g of 3-cyclopropylmethoxy-4-hydroxybenzoic acid with a yield of 95%.
[0080] 1 H NMR (CDCl 3 ): δ0.37-0.40(m, 2H), 0.62-0.77(m, 2H), 1.23-1.40(m, 1H), 3.97(d, 2H), 7.00(d, 1H), 7.63(s, 1H ), 7.75 (d, 1H), 12.1 (br, 1H).
Embodiment 3
[0082] Methyl 3-cyclopropylmethoxy-4-hydroxybenzoate
[0083] Add 19.6 g of 3-cyclopropylmethoxy-4-hydroxybenzoic acid obtained in Example 2 and 200 mL of methanol into the reactor, stir and cool to 0°C. Slowly added 22.4 g of thionyl chloride dropwise, stirred and slowly raised to room temperature, and then heated to reflux for 3 hours. The solvent was evaporated to dryness to obtain 20.9 g of methyl 3-cyclopropylmethoxy-4-hydroxybenzoate with a yield of 99%.
[0084] 1 H NMR (CDCl 3 ): δ0.38-0.41(m, 2H), 0.64-0.77(m, 2H), 1.23-1.43(m, 1H), 3.94(s, 1H), 3.97(d, 2H), 7.09(d, 1H ), 7.63 (s, 1H), 7.70 (d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com