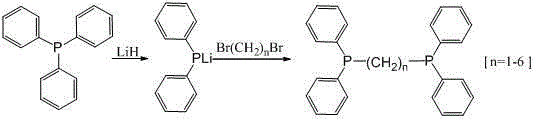Method for synthesizing bis(diphenylphosphino)-alkane
A technology of diphenylphosphine and triphenylphosphine, which is applied in the synthesis of organic phosphine compounds and the field of dialkane compounds, can solve the problem of producing by-products, difficult to control the amount of chloro-tert-butane added, and the inability of ultrasonic irradiation to be widely used in industrialization. and other problems, to achieve the effect of less by-products, easy control of the reaction, and reduction of separation costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Under the protection of argon, add 200 mL THF and 104.9 g (0.4 mol) triphenylphosphine to the dry reactor, then add 3.18 g (0.4 mol) lithium hydride solution in 200 mL THF dropwise under cooling in a water bath, and then stir at room temperature After reacting overnight, after HPLC detected that there was no triphenylphosphine in the reaction system, 62.5 g (0.36 mol) of dibromomethane was added dropwise to the reaction system. After washing with hydrochloric acid, water, and methanol, recrystallize from a mixed solvent of chloroform and methanol to obtain 125.9 g of off-white solid product 1, 1-bisdiphenylphosphine methane, content 98% (HPLC), yield 91%, product melting point 116-118 ℃. Characterization results: GC-MS (EI, m / z): 384 (M + ); 31 P NMR (300MHz, d 6 -DMSO), δ: -23.048; 1 H NMR (300MHz, CDCl 3 ), δ: 7.426-7.352 (s, 20H), 2.912 (s, 2H).
example 2
[0018] Under the protection of argon, 200 mL THF and 104.9 g (0.4 mol) triphenylphosphine were added to the dry reactor, and 3.18 g (0.4 mol) lithium hydride solution in 200 mL THF was added dropwise under cooling in a water bath, and the reaction was stirred at room temperature overnight , after detecting that there is no triphenylphosphine in the reaction system by HPLC, 67.6 g (0.36 mol) 1,2-dibromoethane was added dropwise to the reaction system, and the temperature was raised to reflux after the drop, and the solvent THF was recovered under reduced pressure to obtain a viscous solid , washed with dilute hydrochloric acid, water and methanol in sequence, recrystallized from a mixed solvent of chloroform and methanol to obtain 133.4 g of off-white solid product 1, 2-bisdiphenylphosphineethane, content 98% (HPLC), yield 93%, The melting point of the product is 139-140°C. Characterization results: GC-MS (EI, m / z): 398 (M + ); 31 P NMR (300MHz, d 6 -DMSO), δ: -13.747; 1 H ...
example 3
[0020] Under the protection of argon, add solvent 200 mL THF and 104.9 g (0.4 mol) triphenylphosphine to the dry reactor, drop 3.18 g (0.4 mol) lithium hydride solution in 200 mL THF under cooling in a water bath, and then stir at room temperature After reacting overnight, after HPLC detected that there was no triphenylphosphine in the reaction system, 72.7 g (0.36 mol) of 1,3-dibromopropane was added dropwise to the reaction system. The solid was washed with dilute hydrochloric acid, water, and methanol in sequence, and then recrystallized from a mixed solvent of chloroform and methanol to obtain 132 g of off-white solid product 1, 3-bisdiphenylphosphinopropane, with a content of 98% (HPLC), and a yield of 89%. The melting point of the product is 63-65°C. Characterization results: GC-MS (EI, m / z): 412 (M + ); 31 P NMR (300MHz, d 6 -DMSO), δ: -18.019; 1 H NMR (300MHz, CDCl 3 ), δ: 7.342-7.330 (s, 20H), 2.252-2.198 (t, 4H), 1.589-1.442 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com