In vitro culture method for scatophagus argus kidney cells
A culture method and kidney cell technology, applied to artificial cell constructs, animal cells, vertebrate cells, etc., to achieve strong repeatability and good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation medium
[0020] 1. Basal medium
[0021] Dissolve one packet of L-15 dry powder medium in 800ml of ultrapure water, add 5ml of HEPES (1M) buffer, stir well, adjust the volume to 1L, adjust the pH to 7.4, sterilize with a 0.22μm filter membrane, 4°C Save for later.
[0022] 2. Complete Medium
[0023] Primary medium: Take 100ml of the above basal medium, add 20% FBS, 400U ml -1 Penicillin and 400ug ml -1 streptomycin.
[0024] Primary medium containing bFGF: take 50ml of the above primary medium, add 10ng ml -1 bFGF, formulated as the primary culture medium containing bFGF.
[0025] Subculture medium: use the basal medium prepared above, add 15% and 10% FBS, 100U ml -1 Penicillin and 100ug ml -1 Streptomycin, used up within two weeks.
Embodiment 2
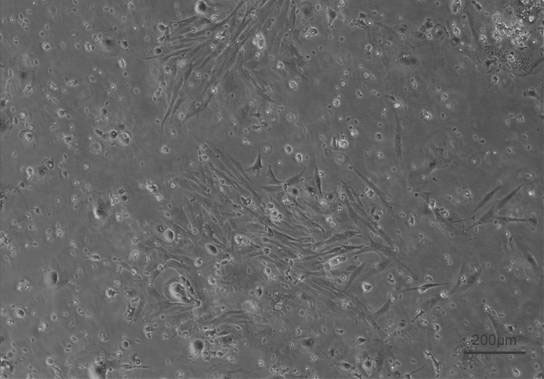
[0026] Example 2 Primary culture of money fish kidney cells
[0027] 1. Sterile kidney extraction
[0028] Take 1-year-old healthy money fish, cut the tail and bleed. The specific operation is as follows: soak the fish body in 75% alcohol for 30s, dissect and remove the kidney tissue in an ultra-clean workbench, and add 400U ml -1 Penicillin and 400ug ml -1 Streptomycin basal medium Wash kidney tissue to remove surface bacteria and blood, in order to ensure tissue sterility, soak kidney tissue in L-15 basal medium containing 5% chlorhexidine, 4% penicillin and streptomycin mixed solution For 5 minutes, cut the tissue to 1mm with ophthalmic scissors 2 size.
[0029] 2. Digestion of isolated kidney cells
[0030] Transfer the shredded kidney tissue to a 15ml centrifuge tube, add 5-6 ml of 0.25% trypsin, digest in a water bath at 28°C for 20 minutes, shake constantly to speed up the separation, and then add complete medium containing 20% FBS Terminate the trypsinization...
Embodiment 3
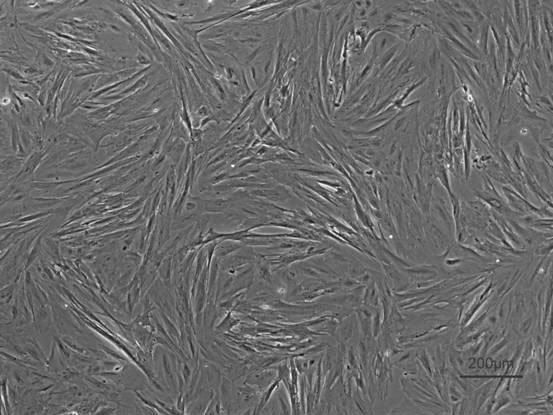
[0033] Example 3 Subculture of money fish kidney cells
[0034] The primary kidney cells covered 80% of the bottom of the bottle and began to be passaged. The specific method of subculture is as follows: first suck out the medium, add 1ml 0.25% trypsin to wash the bottom of the bottle, then pour it out, then add 0.5ml 0.25%-EDTA, place it in a 28°C incubator for 1-2min, and observe the cell changes under the microscope. When round, immediately add 5ml of fresh medium, gently blow the bottom of the bottle to make the adherent cells fall off, the ratio of 1:1 for the first passage, and the ratio of 1:2 for passage thereafter. The concentration of FBS in the 1-10th generation cell culture medium was 15%, and decreased to 10% after the 11th generation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com