Preparation and application for compound for treating cerebrovascular disease
A cerebrovascular disease, compound technology, applied in the field of medicine, to achieve the effect of reducing mortality, reversing ischemic damage of vascular endothelial cells, and enhancing normobaric hypoxia tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
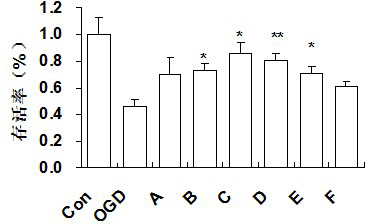
Image
Examples
Embodiment 1
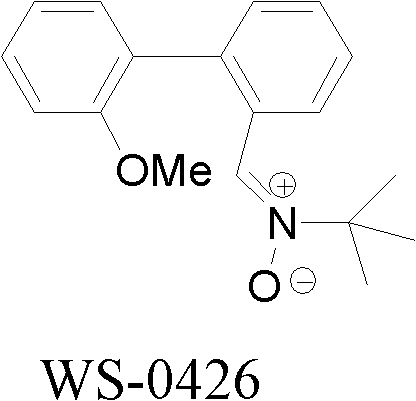
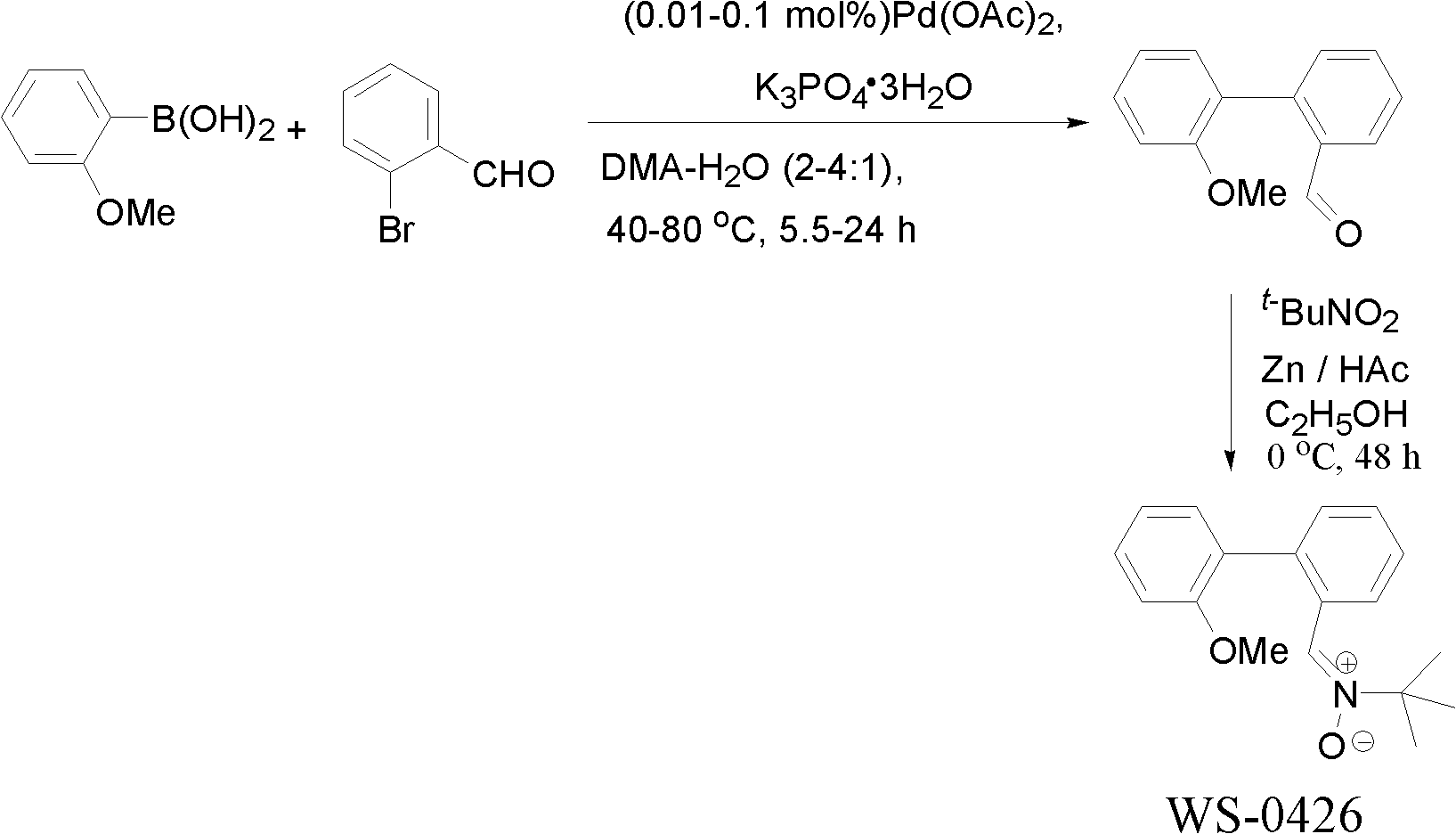
[0022] Example 1 Synthesis of α-[2-(2-methoxyphenyl)]phenyl-N-tert-butylnitrone (WS-0426) by chemical synthesis
[0023] step 1:
[0024] The synthesis of o-(2-methoxyphenyl)benzaldehyde: add 1.85 grams (10mmol, 1.0eq) of o-bromobenzaldehyde, 1.82 grams (12mmol, 1.2eq) of 2-methoxy Phenylboronic acid, 3.2 g (12 mmol, 1.2 eq) of potassium phosphate hydrate, 0.23 mg (0.01 mol%) of Pd(OAc) 2 And 25mL of N, N-dimethylacetamide (DMA): H 2 O (4:1), under the protection of nitrogen, heated to 80°C for 24 hours. After the reaction, lower the temperature to room temperature, add 50mL of water and stir for 5 minutes, extract with ethyl acetate (50mL×3), combine the organic phases, and successively use 10mL of 1N sodium hydroxide aqueous solution, 100mL of water and 100mL of saturated saline Wash each once. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a crude product. The crude product was separated and purifi...
Embodiment 2
[0028] The synthesis of 2-(2-methoxyphenyl)benzaldehyde: the catalyst Pd(OAc) in the embodiment one step 1 2 The dosage was changed to 2.3 mg (0.1 mol%), and the reaction time was changed to 5 hours to obtain 2.04 g of 2-(2-methoxyphenyl)benzaldehyde with a yield of 96%.
Embodiment 3
[0030] The synthesis of 2-(2-methoxyphenyl)benzaldehyde: the catalyst Pd(OAc) in the embodiment one step 1 2 The dosage was changed to 2.3 mg (0.1 mol%), the reaction temperature was changed to 40° C., and the reaction time was changed to 5 hours to obtain 1.95 g of 2-(2-methoxyphenyl)benzaldehyde with a yield of 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com