Fat-soluble composite vitamin I composition freeze-dried powder injection and preparation method thereof
A technology for compound vitamins and vitamins, applied in freeze-dried delivery, drug combination, powder delivery, etc., can solve problems such as difficulty in fully grasping the interaction, difficulty in meeting expectations, and decrease in drug content, so as to be suitable for large-scale production and reduce production costs , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
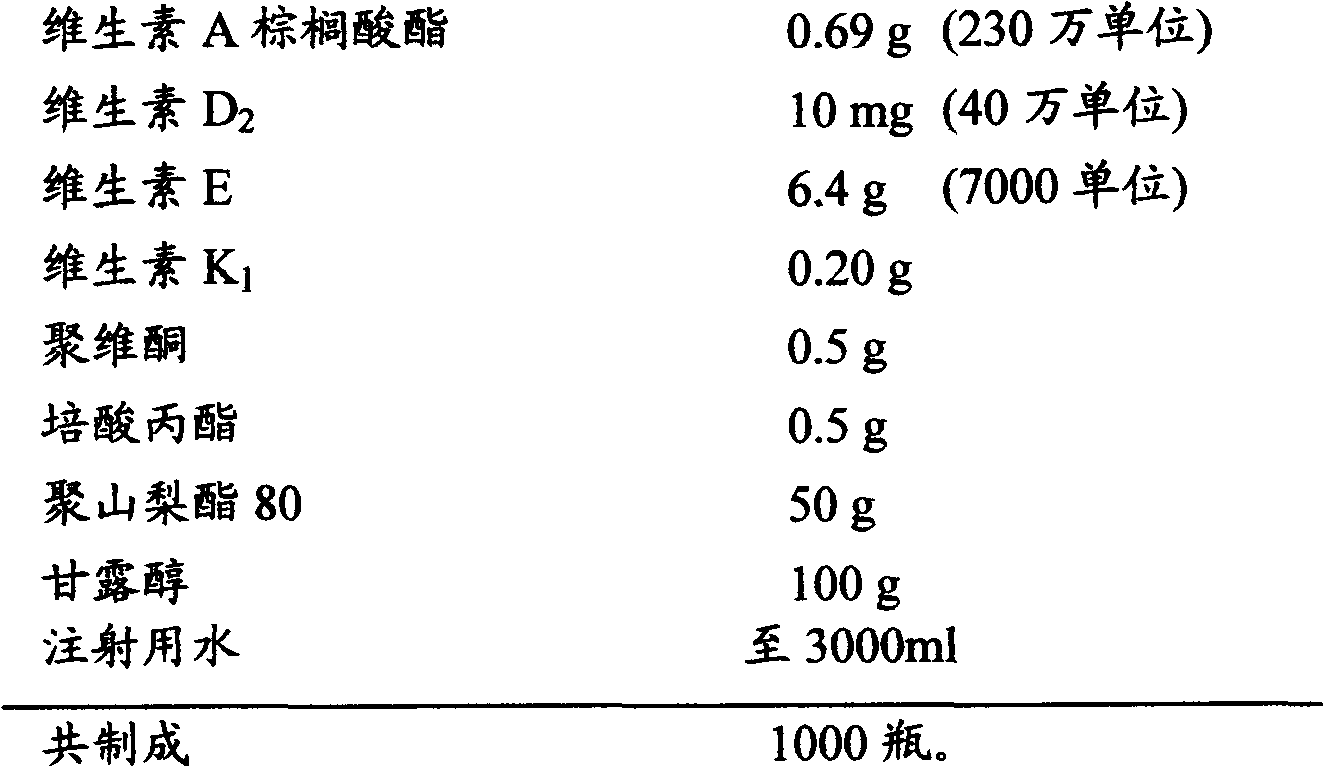
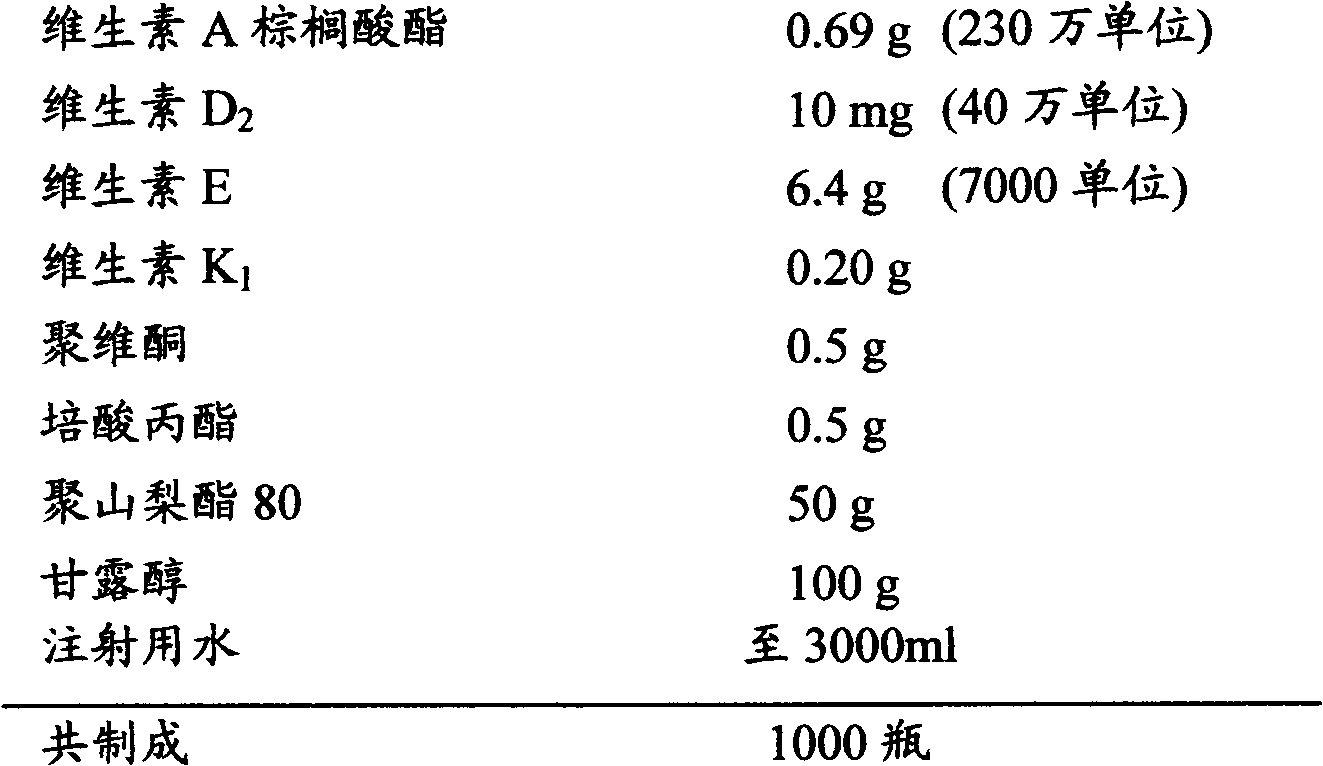
[0021] Raw material composition:
[0022]
[0023] Preparation method: Weigh mannitol, add appropriate amount of water, heat to dissolve, cool, and set aside; weigh povidone, propyl perate, vitamin A palmitate, vitamin K 1 , vitamin E, vitamin D 2 and polysorbate 80, mix well, add water to dissolve, add to the mannitol solution, check the pH value of the liquid (if necessary, use sodium hydroxide or hydrochloric acid solution to adjust the pH value to 6.5-9.0), add water for injection to the full amount, Add 0.01% (g / ml) gac, at room temperature, stir for 30 minutes, filter with a titanium filter stick, filter with a 0.22 μm microporous membrane, and carry out semi-finished product inspection to the gained clear filtrate; Pack; press half the stopper, place in a freeze-drying box, cool down to -40°C, keep warm for 4 hours, turn on the vacuum pump, sublimate and dry, heat up to 30°C and then dry, fill the freeze-drying box with nitrogen, press the stopper, Capping, packagi...
Embodiment 2
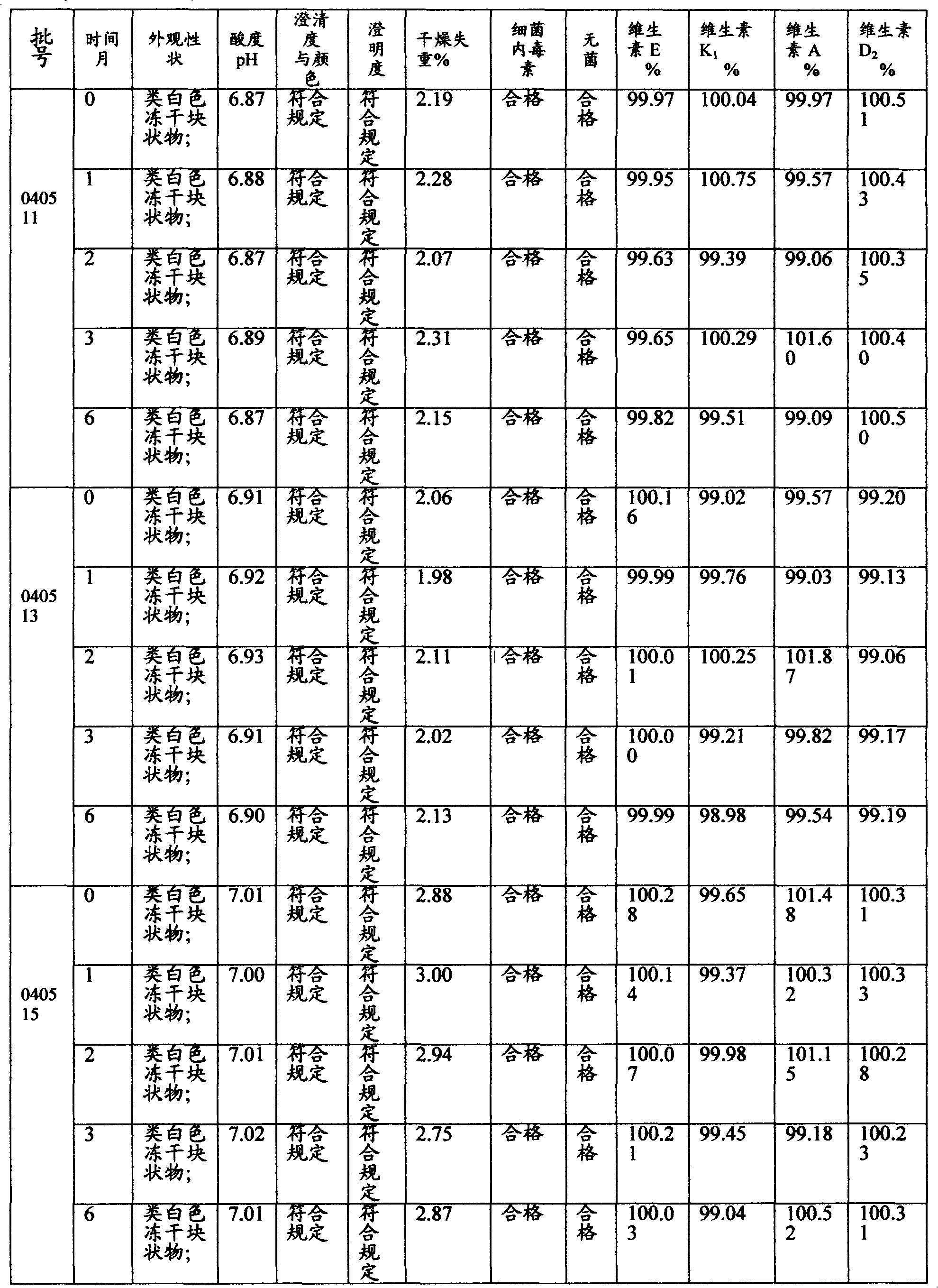
[0024] Embodiment 2: Stability and safety test
[0025] Table 1 Accelerated test results
[0026]
[0027] Table 2 Room temperature test results
[0028]
Embodiment 3
[0029] Embodiment 3 and the contrast of prior art
[0030] The present inventor has compared the preparation of the present invention with the preparations of CN102358081 and CN1903207, that is, the product of their embodiment 1 and the product of the embodiment 1 of the present invention are tested as described in embodiment 2, and the results show that the embodiment of the present invention 1 Product in Vitamin A Palmitate, Vitamin K 1 , vitamin E, vitamin D 2 Content aspect is equivalent or better (not listed in the table) with the product of above-mentioned two kinds of patents, especially after long-term storage i.e. room temperature test 12 months, the preparation of CN102358081, CN1903207 (the following table is referred to as contrast 1 and contrast respectively 2) The detection of visible foreign matter and insoluble particles after reconstitution showed that it was unqualified. This is the biggest difference between it and the product of the present invention. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com