Immunofluorescence dipstick component for quickly and quantitatively detecting protein of plurality of types and detection card component prepared from same and preparation method thereof
A quantitative detection and immunofluorescence technology, which is applied in the field of medical testing, can solve the problems of not being able to meet the requirements of accurate and quantitative clinical diagnosis, not being able to monitor the course of AMI, and being unfavorable for clinical promotion, so as to achieve improved response sensitivity, good specificity, The effect of increasing the dilution factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
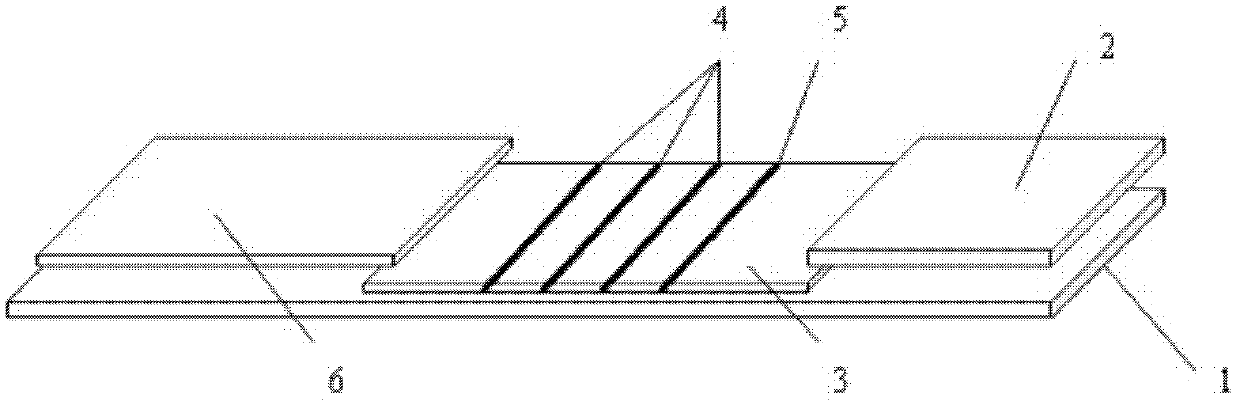
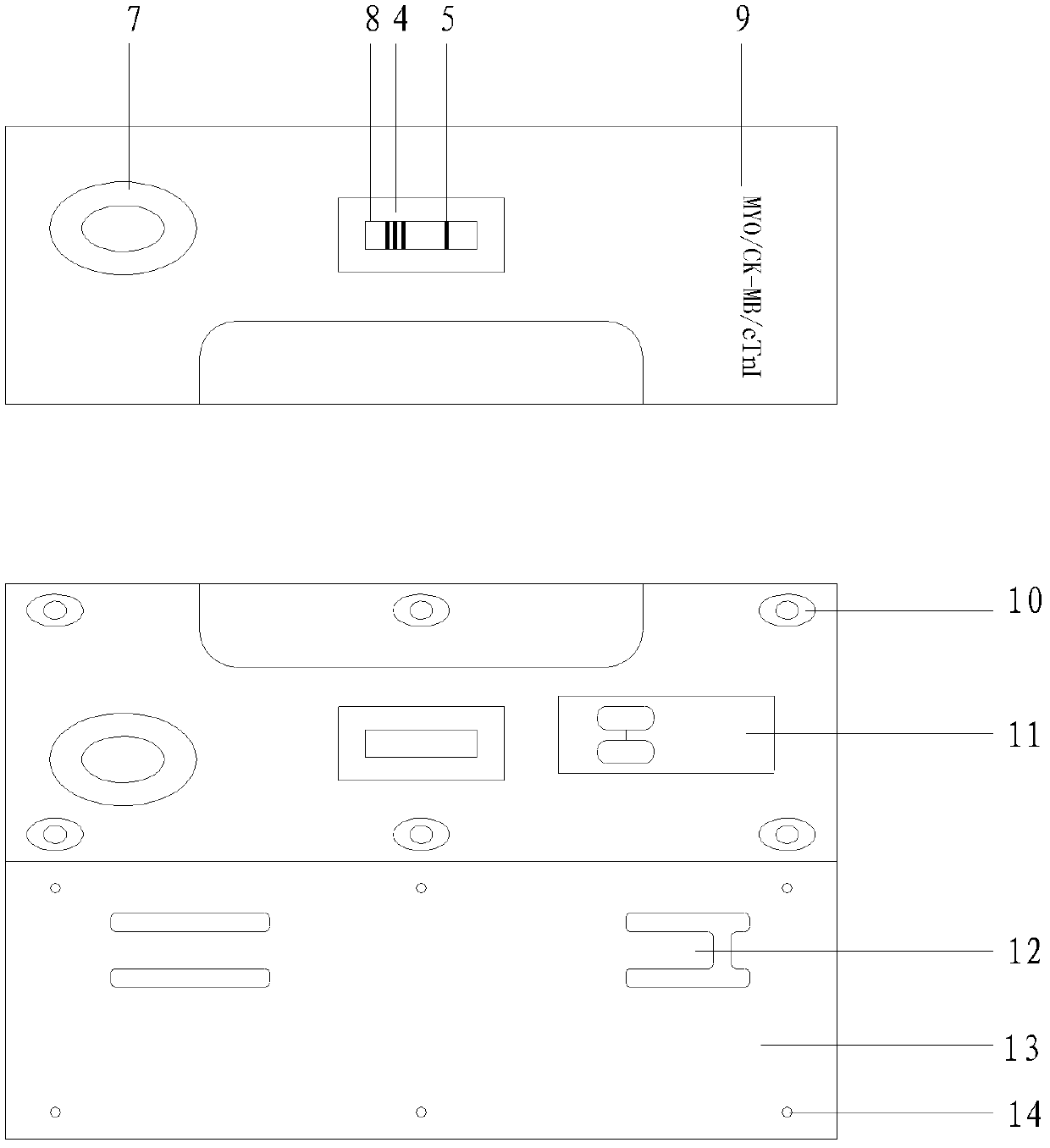
[0059] refer to figure 1 As shown, an immunofluorescence test strip assembly for rapid quantitative detection of various proteins of the present invention is composed of a substrate 1 and a water-absorbing pad 2 sequentially bonded on the substrate 1, a coated analysis membrane 3, and a sample pad 6 The test strip and the independently packaged fluorescein-labeled specific antibody are combined; there are three detection lines 4 and one quality control line 5 on the coating analysis membrane 3, and the three detection lines 4 on the coating analysis membrane respectively coated with an anti-myoglobin monoclonal antibody line, an anti-creatine kinase isoenzyme monoclonal antibody line and a troponin I monoclonal antibody line, the quality control line 5 is coated with rabbit IgG antibody; The independently packaged fluorescein-labeled specific antibodies are separately packaged anti-myoglobin monoclonal antibody, anti-creatine kinase isoenzyme monoclonal antibody, anti-troponin...
Embodiment 2
[0093] The preparation method of the present embodiment is basically the same as that of the first embodiment, the difference is that:
[0094] In step 2, use 50mM pH7.6 phosphate buffer, containing methanol 0.8%, sucrose 1%, bovine serum albumin 0.6% anti-myoglobin monoclonal antibody, anti-creatine kinase isoenzyme monoclonal antibody, anti-myoglobin The concentration of the calpain I monoclonal antibody is 1 mg / m; the preparation method of the quality control line coating buffer is: dilute with polybutene buffer solution of 50mM pH7.6, rabbit containing 0.7% methanol and 0.5% bovine serum albumin The concentration of IgG antibody is up to 0.5mg / ml; the preparation of the coating analysis membrane: adjust the spraying machine, the volume of the membrane liquid is 20ul / 40cm, the machine scribes, the distance between the detection line and the quality control line is 5mm, the scribing is fine and uniform, and it is placed for 25 ℃-37℃ vacuum drying oven for 1.5 hours, bagged a...
Embodiment 3
[0096] The preparation method of the present embodiment is basically the same as that of the first embodiment, the difference is that:
[0097] In step 3, the anti-myoglobin monoclonal antibody, anti-creatine kinase isoenzyme monoclonal antibody, anti-troponin I monoclonal antibody and anti-rabbit IgG antibody were diluted to 1 mg / ml, each take 5ml of antibody solution, add 40mg of fluorescent material platinum porphyrin solution, stir well, incubate at room temperature for 1.5 hours, and mix every 15 minutes. Finally, use a G25 gel column with a specification model for separation and purification, collect the labeled fluorescein-labeled antibody, and use a gel containing 0.01%-0.5% polyethylene glycol, 1%-5% bovine serum albumin, 5%-20% Glycerin and 0.01%-0.05% surfactant are diluted with 0.01M phosphate buffer solution, sealed and packaged in plastic bottles, and stored at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com