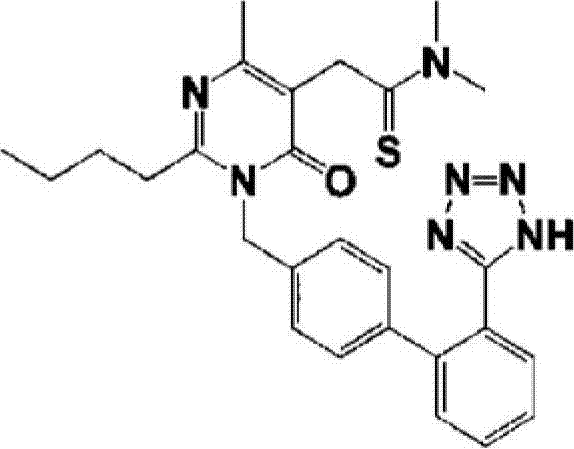
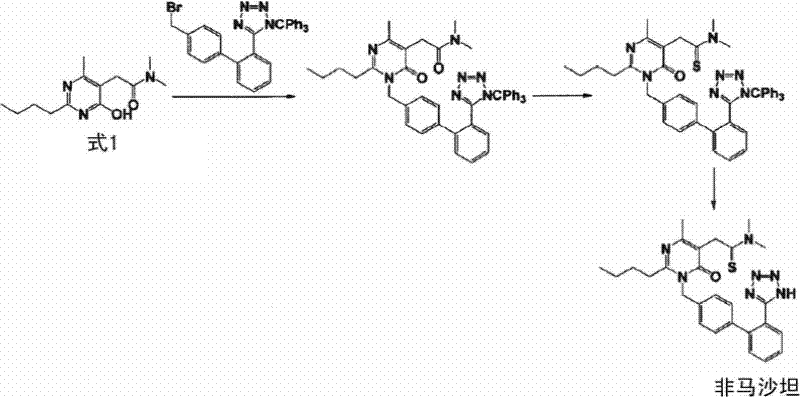
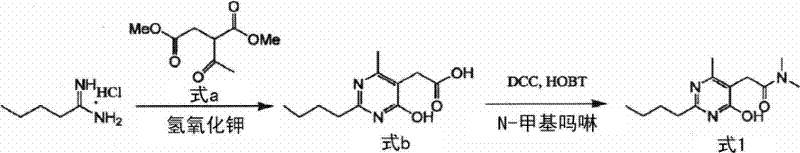
New preparation of 2-(2-n-butyl-4-hydroxy-6-methyl-pyrimidine-5-yl)-n,n-dimethylacetamide
A technology of dimethylacetamide and n-butyl, which is applied in the field of preparing 2--N,N-dimethylacetamide, can solve problems such as difficulty in centrifugation and filtration, achieve easy industrial application, economical preparation method, and increase yield rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0063] Preparation Example 1: Preparation of 2-(N,N-dimethylaminocarbonylmethyl)-methyl acetoacetate
[0064] In an ice bath, methyl acetoacetate (11.61 g, 0.1 mol) was dissolved in methanol (60 mL), and sodium methoxide (5.67 g, 0.105 mol) was added thereto, followed by stirring for 30 minutes. 2-Chloro-N,N-dimethylacetamide (12.40 g, 0.1 mol) was added dropwise thereto over 30 minutes, and the ice bath was removed, followed by stirring under reflux for 5 hours. The reaction mixture was cooled to 20° C., the solvent was removed under vacuum, and 100 mL of chloroform and 100 mL of purified water were added thereto, followed by stirring and separating the organic layer. The organic layer was concentrated, and the residue was purified by chromatography using a 1:2 (v / v) mixed solution of ethyl acetate and n-hexane to provide 12.06 g (yield: 57.1%) of a pale yellow transparent oil.
[0065] 1 H-NMR (200MHz, CDCl 3 )d 2.40(s, 1H), 2.91(s, 3H), 3.04(s, 3H), 3.10-2.75(m, 2H), 3.7...
preparation example 2
[0066] Preparation Example 2: Preparation of 2-(N,N-dimethylaminocarbonylmethyl)-ethyl acetoacetate
[0067] In an ice bath, ethyl acetoacetate (16.92 g, 0.130 mol) was dissolved in absolute ethanol (90 mL), and sodium ethoxide (9.78 g, 0.137 mol) was added thereto, followed by stirring for 30 minutes. 2-Chloro-N,N-dimethylacetamide (16.13 g, 0.130 mol) was added dropwise thereto, and the ice bath was removed, followed by stirring at room temperature for 15 hours. The solvent was removed under reduced pressure, and 150 mL of chloroform and 150 mL of purified water were added thereto, followed by stirring and separating the organic layer. The organic layer was concentrated, and the residue was purified by chromatography using a 1:5 (v / v) mixed solution of ethyl acetate and n-hexane to provide 12.96 g (yield: 41.0%) of a colorless transparent oil.
[0068] 1 H-NMR (200MHz, CDCl 3 )d 1.08(t, 3H), 1.20(t, 3H), 1.30(t, 3H), 2.40(s, 2H), 2.80(dd, 1H), 3.04(dd, 1H), 3.34(m, 4H) ,...
Embodiment 1
[0069] Example 1: Preparation of 2-(2-n-butyl-4-hydroxyl-6-methyl-pyrimidin-5-yl) using 2-(N,N-dimethylaminocarbonylmethyl)-methyl acetoacetate -N,N-Dimethylacetamide
[0070] Pentaneamidine hydrochloride (5.96 g, 43.6 mmol) was dissolved in 60 mL of ethanol, and 2-(N,N-dimethylaminocarbonylmethyl)-methyl acetoacetate obtained in Preparation Example 1 was added thereto ( 8.77g, 43.6mmol) and potassium hydroxide (2.88g, 43.6mmol), then stirred at 25°C for 15 hours. Concentration was performed under vacuum, and 50 mL of chloroform and 50 mL of purified water were added to the concentrate, followed by stirring and standing to separate an organic layer. The organic layer was concentrated, and 10 mL of ethyl acetate and 50 mL of hexane were added thereto, followed by reflux, cooling and filtration. The obtained filtrate was washed with 10 mL of a 1:5 (v / v) mixed solvent of ethyl acetate:hexane, and then dried to provide 7.7 g (30.6 mmol, yield: 70%) of the title compound as a whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com