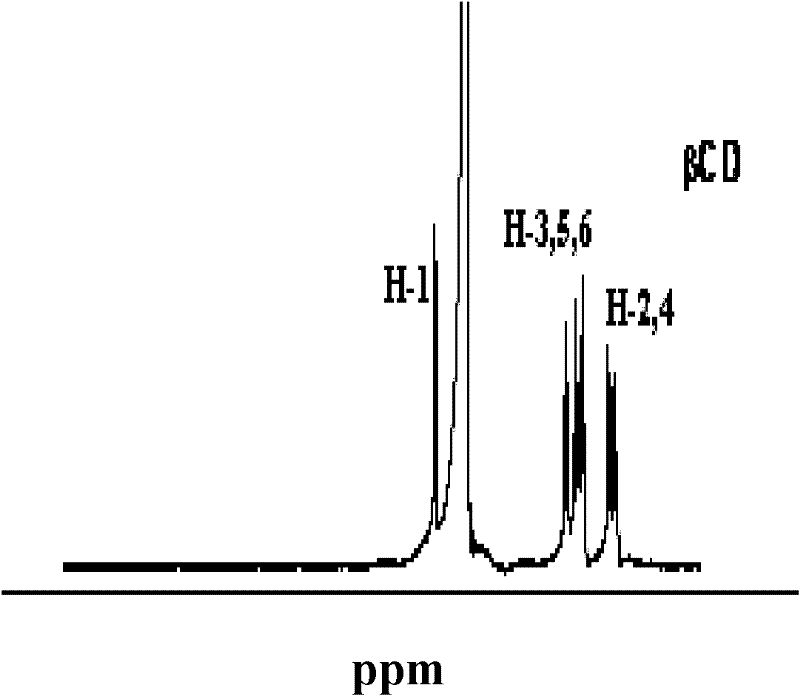
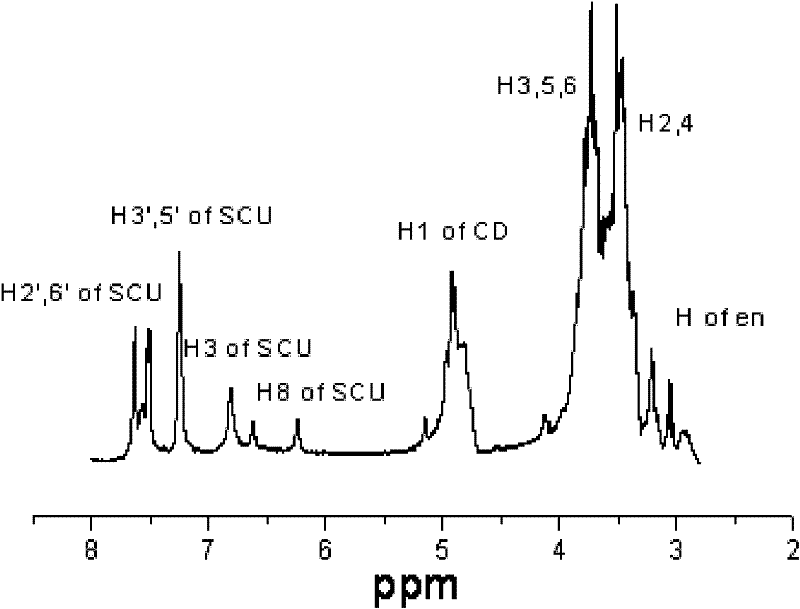
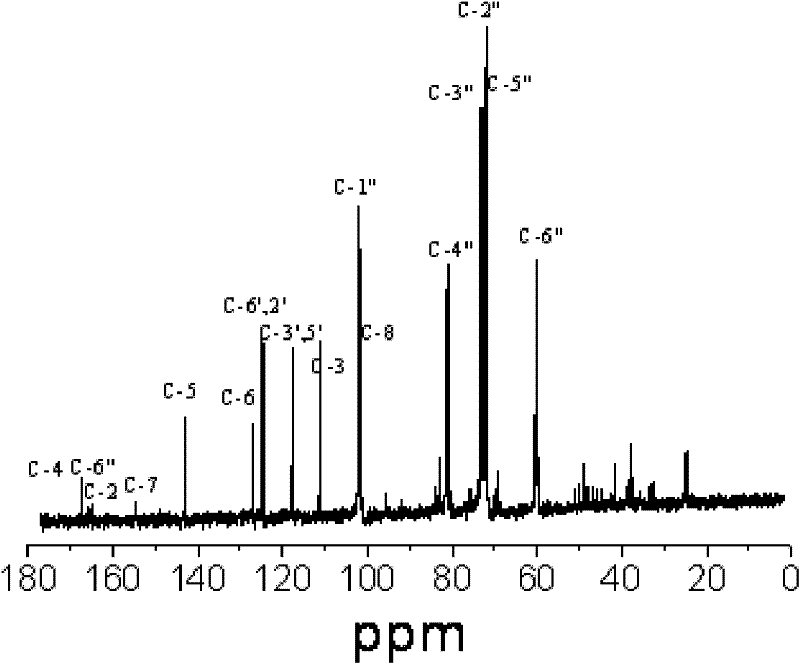
Scutellarin prodrug and preparation method thereof
A technology of scutellarin and prodrugs, which is applied in the field of scutellarin prodrugs and its preparation, can solve the problems of poor selective release characteristics, etc., achieve improved bioavailability, avoid release and destruction, and have good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Preparation of sulfonylated cyclodextrin
[0058] Refer to existing literature [R.C.Petter, J.S.Salek, C.T.Sikorski, G.Kumaravel, and F.-T.Lin: J.Am.Chem.Soc.112, 3860-3868 (1990)].
[0059]Take 210g of the recrystallized β-cyclodextrin, dissolve it in 1300mL of distilled water, and stir the solution into a white emulsion, add sodium hydroxide solution (17.2g, 50mL), and stir for 1.5h. Weigh 26.0g of p-toluenesulfonyl chloride, dissolve it in 80mL of acetonitrile solution, slowly add the solution dropwise to β-cyclodextrin lye, stir at room temperature for 2 hours, remove a small amount of insoluble matter by suction filtration, and adjust the pH value of the filtrate with 2M hydrochloric acid To 7.5, at this time a large amount of precipitation occurs, and the filtrate is removed by suction filtration. Dissolve the precipitate in 450mL of water under heating, filter out the insoluble matter while it is hot, recrystallize the filtrate at 0°C for 12 hours, recrystall...
Embodiment 2
[0067] Dissolve 2.61g (2mmol) of mono-[2-(ethylenediamino)-6-deoxy]-β-cyclodextrin in anhydrous N,N-dimethylamide (50mL) and cool to about 0°C . Add 1.2 g of dicyclohexylcarbodiimide (DCC) and 0.9 g of 1-hydroxybenzotriazole (HOBT) to this solution, add scutellarin 2.77 g (6 mmol) after stirring in an ice bath for 5 h, and the reaction solution is Stir in ice bath for 20 h, and stir at room temperature for 10 h (using TCL tracking detection, developer: methanol: ethyl acetate: water = 7:7:1). The reaction solution was evaporated to dryness under reduced pressure at 60°C, the residue was fully dissolved in water, fully dissolved in water, filtered, the filtrate was concentrated, dropped into 100mL chloroform for filtration, the precipitate was collected, and vacuum-dried at 50°C for 24 hours to obtain 2-scutellarin bonded β-cyclodextrin, 65% yield.
Embodiment 3
[0069] Dissolve 2 mmol of mono-[3-(methylamino)-6-deoxy]-α-cyclodextrin in anhydrous DMF (50 mL), and cool to about 0°C. Add 0.4 g of dicyclohexylcarbodiimide and 0.3 g of 1-hydroxybenzotriazole to the solution, stir in an ice bath for 3 h, then add 2.77 g (6 mmol) of scutellarin, and stir the reaction solution in an ice bath for 6 h. Stir for 12 h (using TCL tracking detection, the developer is methanol: ethyl acetate: water = 7:7:1). The reaction solution was evaporated to dryness under reduced pressure at 60°C, the residue was fully dissolved in 50g of water, filtered, the filtrate was concentrated, 100mL of ethanol was added dropwise, filtered, the precipitate was collected, and vacuum-dried at 50°C for 24 hours to obtain 3-scutellarin bonded α - Cyclodextrin, yield 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com