Cu-Ni functionally gradient material and preparation method thereof
A gradient material, cu-ni technology, used in chemical instruments and methods, layered products, metal layered products, etc., can solve the problem of inability to prepare high-temperature oxidation resistance, electrical conductivity, thermal conductivity and mechanical properties of materials, loss of electrical conductivity of materials and mechanical toughness, the inability to prepare alloy surfaces, etc., to achieve the effects of good electrical conductivity, strong surface oxidation resistance, and tight bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
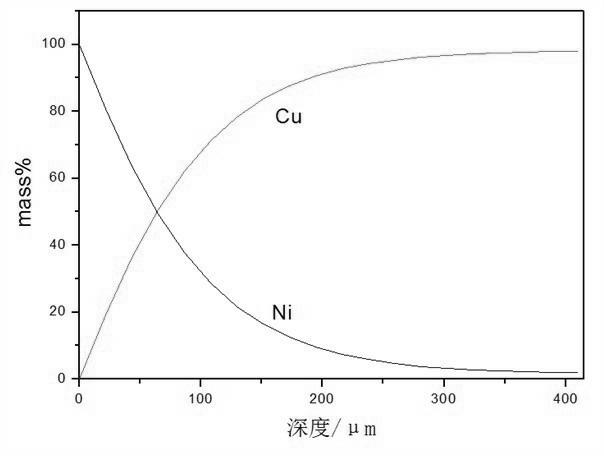
[0024] Embodiment 1: This Cu-Ni gradient material is prepared by the following process.
[0025]Prepare molten salt according to the proportion of 0.2mol NaCl, 0.2mol KCl, 0.6mol KF, and NiO as 1.5 times the saturation amount, put the above molten salt system into a graphite crucible, put it into an electric furnace and raise the temperature to 650°C, and keep it at a constant temperature for 60 minutes. NiO dissolves in the molten salt to reach saturation; put the cathode copper plate into the molten NaCl-KCl-KF-NiO molten salt, apply a DC pulse current with a current density of 150 cm -2 At the same time, after 10 minutes of electrodeposition, take out the cathode material from the molten NaCl-KCl-KF-NiO molten salt system, put it into boiling water and cook for about 30 minutes, so that there is no obvious molten salt attachment on the copper plate substrate, and then use deionized water, Rinse the sample with alcohol. The analysis results of the coating surface morpholo...
Embodiment 2
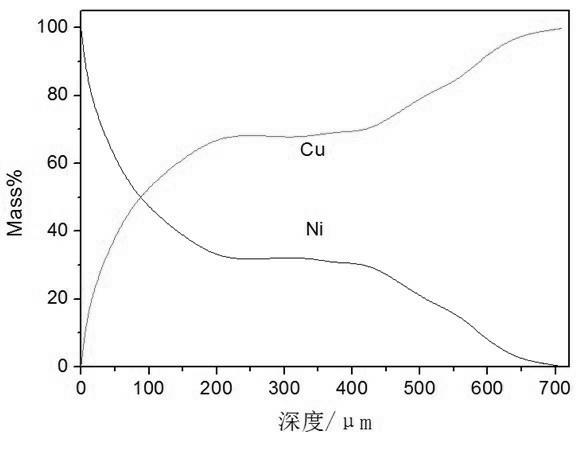
[0026] Embodiment 2: This Cu-Ni gradient material is prepared by the following process.
[0027] Prepare molten salt according to the molar fraction of 0.3mol NaCl, 0.3mol KCl, 0.4mol KF, and NiO as twice the saturation amount, put the above molten salt system into a graphite crucible, put it into an electric furnace and raise the temperature to 700°C, and keep the temperature for 120min. NiO dissolves in the molten salt to reach saturation; put the cathode copper plate into the molten NaCl-KCl-KF-NiO molten salt, apply a DC pulse current with a current density of 200 cm -2 At the same time, after 20 minutes of electrodeposition, take out the cathode material from the molten NaCl-KCl-KF-NiO molten salt system, put it in boiling water and cook for about 10 minutes, so that there is no obvious molten salt attachment on the copper plate substrate, and then use deionized water, Rinse the sample with alcohol. The analysis results of the coating surface morphology, section thickn...
Embodiment 3
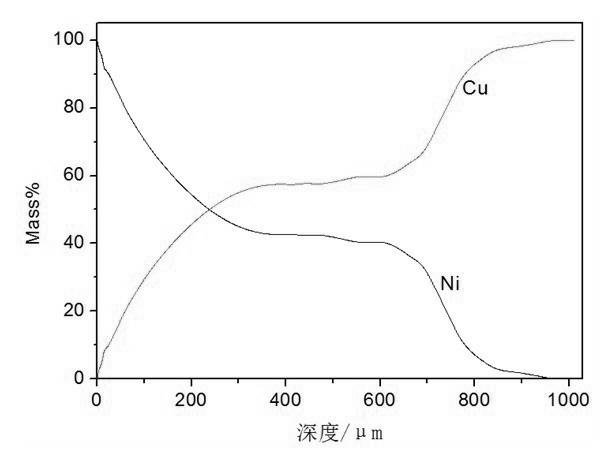
[0028] Embodiment 3: This Cu-Ni gradient material is prepared by the following process.
[0029] Prepare molten salt according to the ratio of 0.4mol NaCl, 0.4mol KCl, 0.2mol KF, and NiO to 3 times the saturation amount, put the above molten salt system into a graphite crucible, put it into an electric furnace and raise the temperature to 750°C, and keep the temperature for 90 minutes. NiO dissolves in the molten salt to reach saturation; put the cathode copper plate into the molten NaCl-KCl-KF-NiO molten salt, apply a DC pulse current with a current density of 250 cm -2 At the same time, after 30 minutes of electrodeposition, take out the cathode material from the molten NaCl-KCl-KF-NiO molten salt system, put it into boiling water and cook for about 20 minutes, so that there is no obvious molten salt attachment on the copper plate substrate, and then use deionized water, Rinse the sample with alcohol. The analysis results of the coating surface morphology, section thickne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com