Environment-friendly method for preparing water-soluble grapheme at normal temperature
A water-soluble, alkene-green technology, applied in graphene, nano-carbon and other directions, can solve the problems of inability to mass-produce graphene dispersibility and water-solubility, high reaction temperature, environmental hazards, etc., and achieve good water solubility and stable dispersion. the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
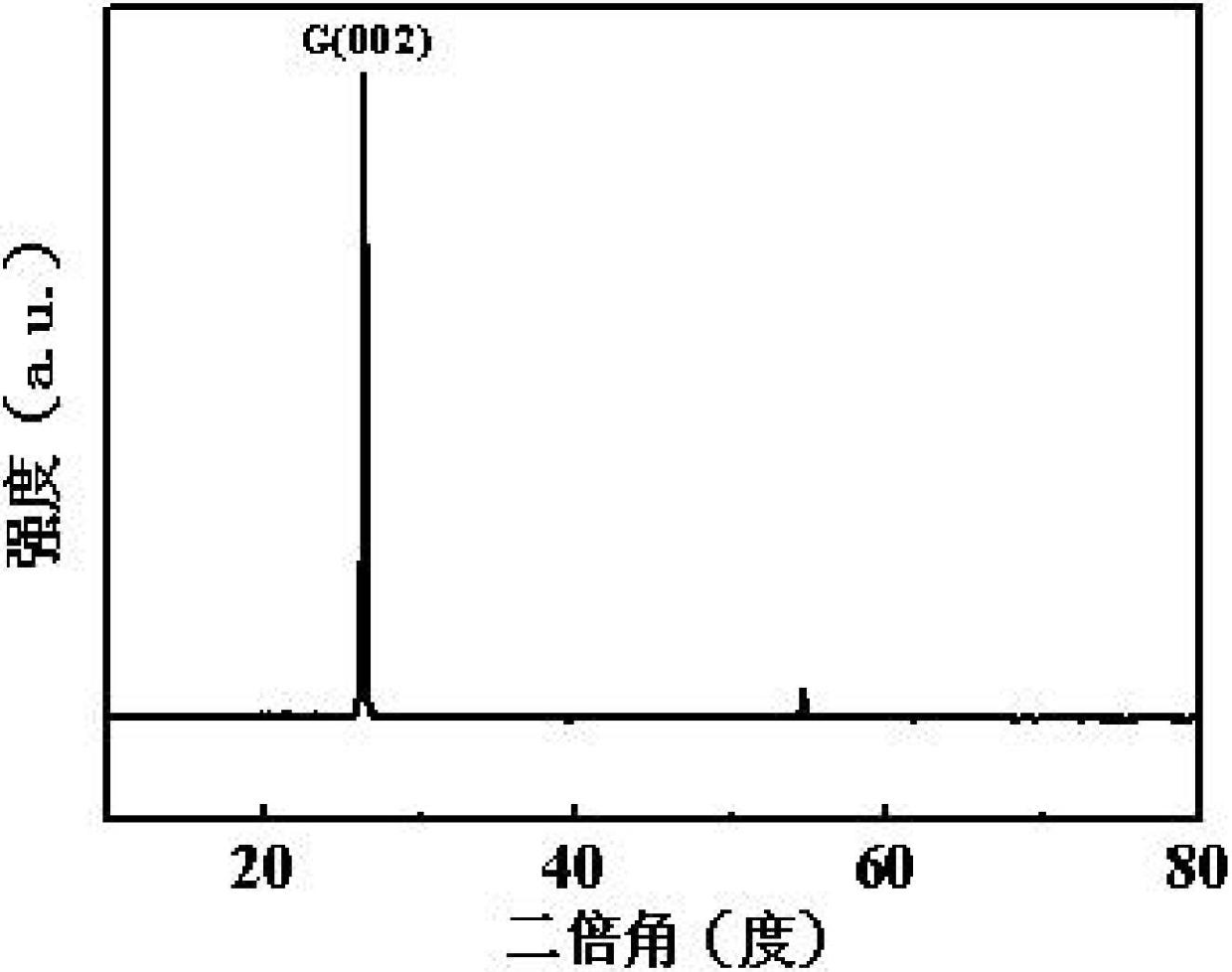
Image
Examples
specific Embodiment approach 1
[0036] Specific embodiment one: the green preparation method of water-soluble graphene at normal temperature in this embodiment is carried out according to the following steps:
[0037]One, take by weighing graphite powder, concentrated sulfuric acid, sodium nitrate, potassium permanganate, deionized water; Wherein the ratio of graphite powder and the vitriol oil is 1g: (20~80mL); The ratio of graphite powder and sodium nitrate is 1g: ( 0.5~2.0g); The ratio of graphite powder and potassium permanganate is 1g: (3~10g); The ratio of graphite powder and deionized water is 1g: (100~200mL); The mass fraction of concentrated sulfuric acid is 98%;
[0038] 2. Place the dry beaker in an ice bath, add the graphite powder, concentrated sulfuric acid and sodium nitrate weighed in step 1 to the beaker, stir and mix evenly, then add potassium permanganate, and control the reaction temperature not to exceed 10°C. Continue magnetic stirring for 2 to 3 hours to complete the exfoliation of gr...
specific Embodiment approach 2
[0046] Specific embodiment two: this embodiment is different from specific embodiment one: the ratio of graphite powder and the vitriol oil in step 1 is 1g: (40~70mL); The ratio of graphite powder and sodium nitrate is 1g: (1.0~1.5 g); the ratio of graphite powder to potassium permanganate is 1g: (5-8g); the ratio of graphite powder to deionized water is 1g: (120-180mL). Others are the same as in the first embodiment.
specific Embodiment approach 3
[0047] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the reaction temperature in step 2 is 5-8°C. Others are the same as in the first or second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com