Method for synthesizing alpha-amino-acid ester
An amino acid ester, ketoester technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems affecting the application of imine hydrogenation reaction, and achieve the expansion of substrate application scope, large industrialization potential, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
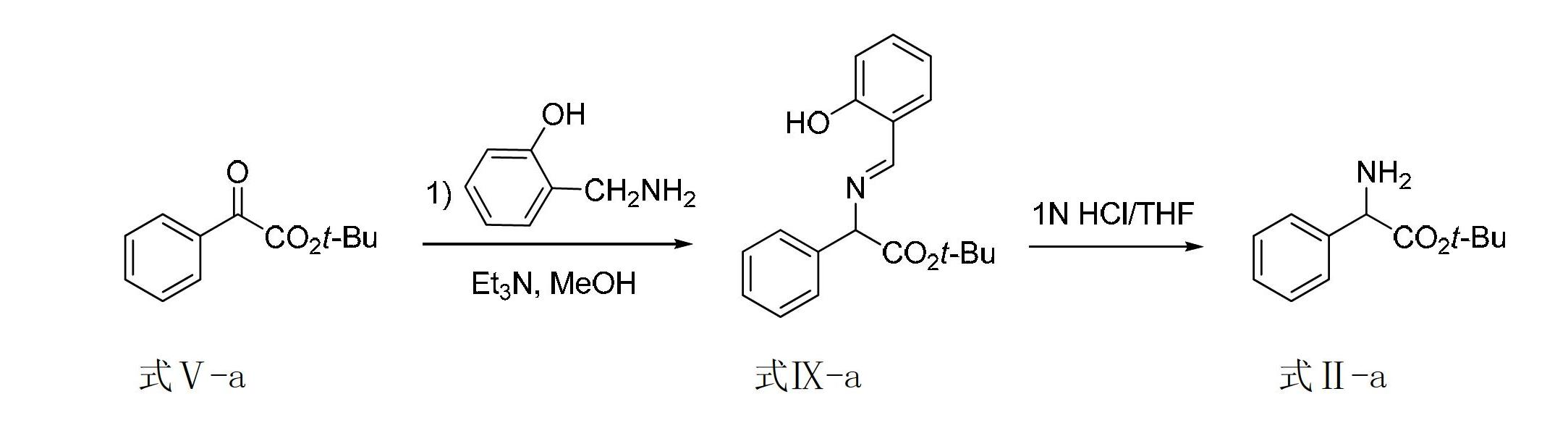
[0069] Embodiment 1, synthetic 2-amino-tert-butyl phenylacetate
[0070] For the synthetic route map, see figure 2 .
[0071]
[0072] Formula Ⅴ-a Formula IX-a Formula Ⅱ-a
[0073] 1) Add tert-butyl benzoate (0.60mmol, 0.124g), 2-hydroxybenzylamine (1.20mmol, 0.148g), triethylamine (0.12mmol, 0.012g) and 3.0mL methanol to the reactor in sequence. Put the reactor into an oil bath at 60°C, and after reacting for 24 hours, take the reactor out from the oil bath, and spin dry the solvent methanol to obtain the transamination crude product shown in formula IX-a, with a conversion rate of 100 as determined by NMR. %, without further purification, the next reaction was carried out directly.
[0074] 2) Dissolve the transamination crude product obtained from the above reaction in a mixed solution of 12.0 mL tetrahydrofuran and 1 mol / liter hydrochloric acid aqueous solution (the volume ratio of tetrahydrofuran to hydrochloric acid aqueous solution is 1:1), and the temperature of...
Embodiment 2
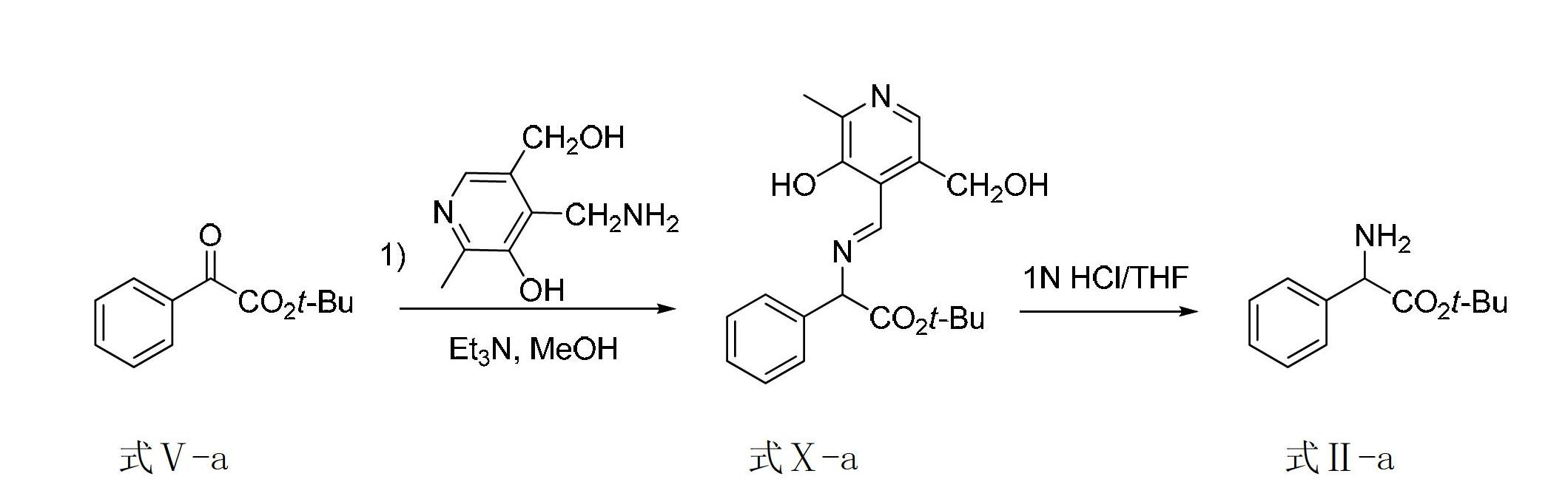
[0077] Embodiment 2, synthetic 2-amino-tert-butyl phenylacetate
[0078] For the synthetic route map, see image 3 .
[0079]
[0080] Formula Ⅴ-a Formula Ⅹ-a Formula Ⅱ-a
[0081] 1) Add tert-butyl benzoate (0.30mmol, 0.062g), pyridoxamine (0.36mmol, 0.061g), triethylamine (0.06mmol, 0.006g) and 1.5mL methanol to the reactor in sequence. Put the reactor into an oil bath at 60°C, and after reacting for 6 hours, take the reactor out from the oil bath, and spin dry the solvent methanol to obtain the transamination crude product shown in formula Ⅹ-a, and the conversion rate as determined by NMR is 100 %, without further purification, the next reaction was carried out directly.
[0082] 2) Dissolve the transaminated crude product obtained in the above reaction in a mixed solution of 6.0mL tetrahydrofuran and 1 mol / liter hydrochloric acid aqueous solution (the volume ratio of tetrahydrofuran to hydrochloric acid aqueous solution is 1:1), and the temperature of the mixture is a...
Embodiment 3
[0085] Embodiment 3, synthesis 2-amino-2-cyclohexyl-tert-butyl acetate
[0086] For the synthetic route map, see Figure 4 .
[0087]
[0088] Formula Ⅳ-a Formula Ⅺ-a Formula Ⅰ-a
[0089] 1) Add tert-butyl cyclohexyl ketoate (0.60mmol, 0.127g), 2-hydroxybenzylamine (1.20mmol, 0.148g), triethylamine (0.12mmol, 0.012g) and 3.0mL methanol to the reactor in sequence . Put the reactor in an oil bath at 60°C, take it out from the oil bath after reacting for 48 hours, and spin dry the solvent methanol to obtain the transamination crude product shown in formula Ⅺ-a, which can be directly processed without further purification Next reaction.
[0090] 2) Dissolve the transamination crude product obtained from the above reaction in a mixed solution of 12.0 mL tetrahydrofuran and 1 mol / liter hydrochloric acid aqueous solution (the volume ratio of tetrahydrofuran to hydrochloric acid aqueous solution is 1:1), and the temperature of the mixture is at room temperature under hydrolysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com