Preparation method of nano dysprosium titanate powder
A nano-titanium and powder technology, applied in nanotechnology, chemical instruments and methods, titanium compounds, etc., can solve the problems of easy incorporation of impurities, large particle size of synthetic powder, and achieve uniform particle size, low crystal formation temperature, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
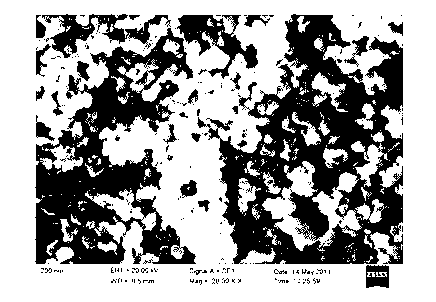
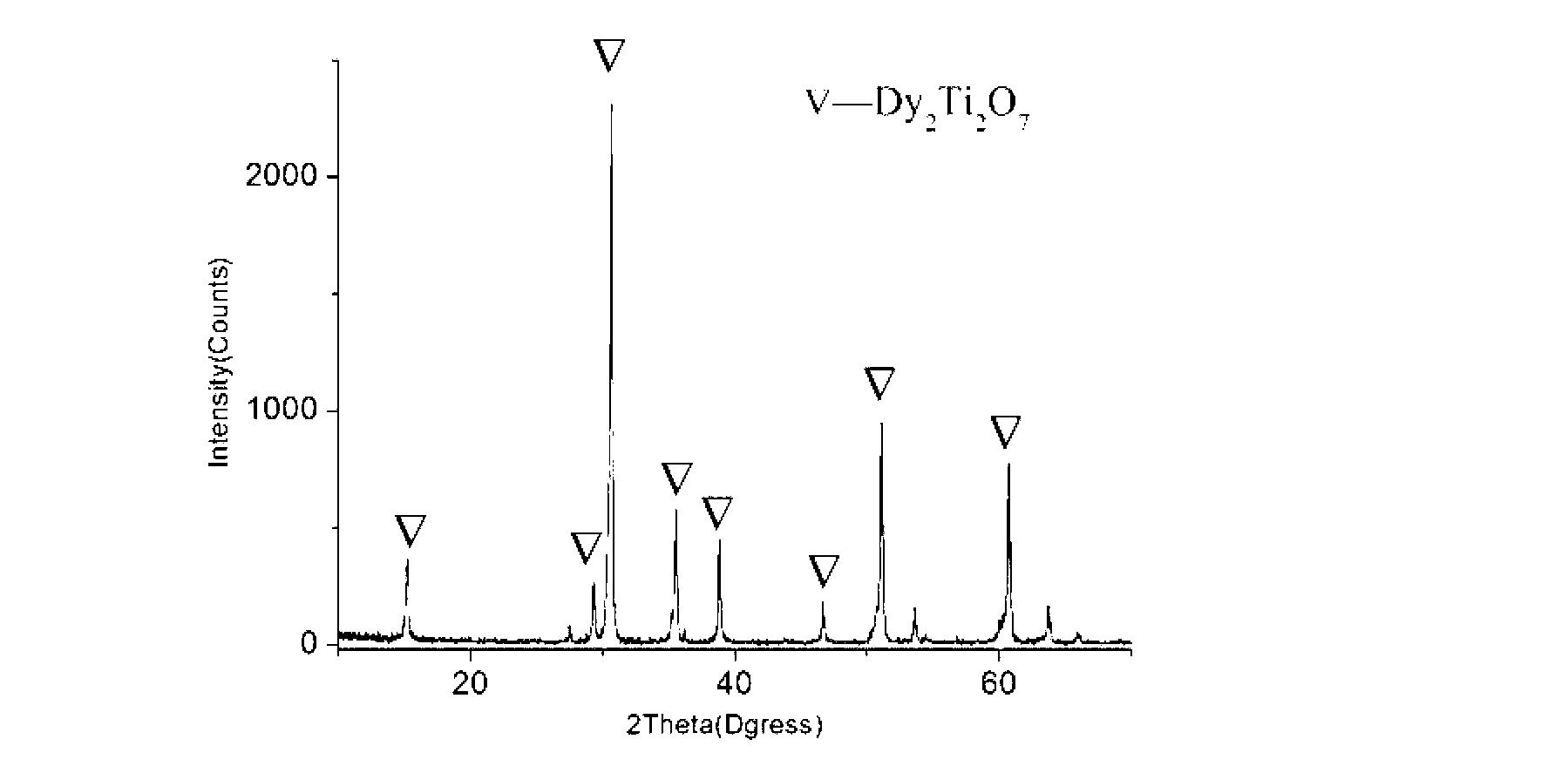
Image
Examples
Embodiment 1
[0026] Example 1: Molar ratio Dy:Ti=1:1
[0027] 1. Add 40ml of butyl titanate to 20ml of absolute ethanol while stirring to obtain a butyl titanate alcohol solution;
[0028] 2. Add 30ml of absolute ethanol to 10ml of deionized water and stir evenly, add hydrochloric acid to adjust the pH to 2; add 4ml of glacial acetic acid to slow down the hydrolysis and polymerization speed of butyl titanate, then add 41g of dysprosium nitrate to the solution, stir to make It is completely dissolved to obtain dysprosium nitrate solution;
[0029] 3. Slowly add the dysprosium nitrate solution in step 2 into the butyl titanate solution in step 1, and stir fully to obtain a mixed sol;
[0030] 4. Aging the mixed sol prepared in step 3 at room temperature for 24 hours to obtain a gel; then drying the gel at 80°C, pulverizing, and passing through a 100-mesh sieve to obtain a dry gel powder of dysprosium titanate precursor;
[0031] 5. Put the dysprosium titanate precursor xerogel into an alum...
Embodiment 2
[0033] Embodiment 2: molar ratio Dy:Ti=1.7:1
[0034] 1. Add 40ml of butyl titanate to 40ml of absolute ethanol while stirring to obtain a butyl titanate solution;
[0035] 2. Add 30ml of absolute ethanol to 20ml of deionized water and stir evenly, add hydrochloric acid to adjust the pH to 1.5; add 2ml of glacial acetic acid to slow down the hydrolysis and polymerization speed of butyl titanate, the molar ratio Dy:Ti is 1.7:1, Add 69.7g of dysprosium nitrate in the solution, stir to make it dissolve completely, and obtain dysprosium nitrate solution;
[0036] 3. Slowly add the dysprosium nitrate solution in step 2 into the butyl titanate solution in step 1, and stir fully to obtain a mixed sol;
[0037] 4. Aging the mixed sol prepared in step 3 at room temperature for 24 hours to obtain a gel; then drying the gel at 50°C, pulverizing, and passing through a 200-mesh sieve to obtain a dry gel powder of dysprosium titanate precursor;
[0038] 5. Put the dysprosium titanate prec...
Embodiment 3
[0040] Embodiment 3: molar ratio Dy:Ti=1.2:1
[0041] 1. Add 40ml of butyl titanate to 20ml of absolute ethanol while stirring to obtain a butyl titanate solution;
[0042] 2. Add 20ml of absolute ethanol to 10ml of deionized water and stir evenly, add hydrochloric acid to adjust the pH to 0.5~1; add 3ml of glacial acetic acid to slow down the hydrolysis and polymerization speed of butyl titanate, and the molar ratio Dy:Ti is 1.2: 1. Add 49g of dysprosium nitrate to the solution, stir to make it completely dissolve, and obtain dysprosium nitrate solution;
[0043] 3. Slowly add the dysprosium nitrate solution in step 2 into the butyl titanate solution in step 1, and stir fully to obtain a mixed sol;
[0044]4. Aging the mixed sol prepared in step 3 at room temperature for 24 hours to obtain a gel; then drying the gel at 70°C, pulverizing, and passing through a 100-mesh sieve to obtain a dysprosium titanate precursor xerogel powder;
[0045] 5. Put the dysprosium titanate pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com