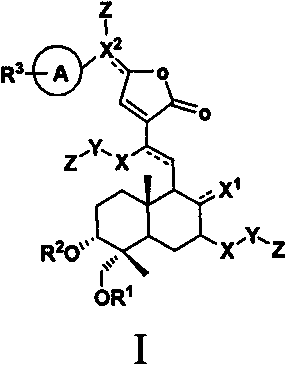
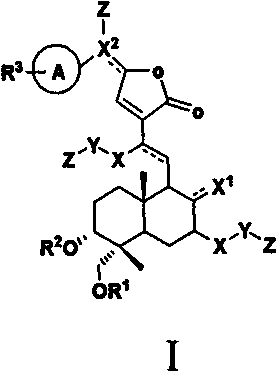
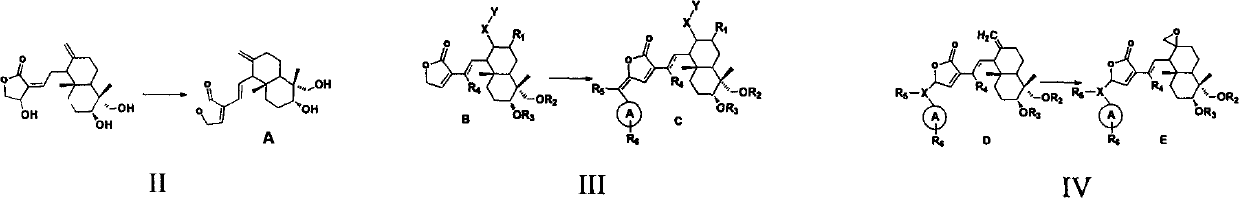
Andrographolide analogue and application of andrographolide analogue to treatment
A kind of technology of andrographolide, analog, applied in the field of new andrographolide analog
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] Synthesis and preparation example (see 1-436 Table 1 for example chemical structure)
[0033] Andrographolide was purchased from China Huatai Biotechnology Co., Ltd., and the H NMR spectrum was determined by using 1 HNMR (600M Hz, DMSO-d 6 ).
[0034] General reaction A (hydroxyl esterification)
[0035] Andrographolide 3.00 grams (10mmol) and DMAP1.2 grams (10mmol), triethylamine 1.5 grams (15mmol) are mixed in the methylene chloride of 20 milliliters, add 4.8 grams (15mmol) of 4-O glucosyl benzoyl chloride respectively , stirring until the reaction is complete, the reaction solution is filtered and separated by silica gel column chromatography to obtain the target compound.
[0036] General reaction B (hydroxyl deprotection)
[0037] Acetylated protected andrographolide 4.30 g (10 mmol) was mixed with 20 ml of ethanol and 5 ml of water mixed solution, 1.50 g of potassium carbonate was added, the mixture was heated to reflux for 2 hours, the reaction solution was f...
Embodiment 23
[0044] Example 2.3, Preparation of 14,19-triacetyl andrographolide: 3.00 g (8.6 mmol) of andrographolide was dissolved in 20 ml of acetic anhydride, 2.0 g (0.3 mmol) of zinc chloride was added, and the mixture was reacted at 80° C. for 5 h. The reaction solution was extracted with ethyl acetate and separated by silica gel column chromatography to obtain the target product; IR (KBr, cm -1 ): 3436-3078, 1753, 1736, 1728, 1247, 1132, 1095, 1077; 1 HNMR (CDCl 3 ): δ6.89(t, 1H), 5.93(d, J=6Hz, 1H), 4.74(br, 2H), 4.56(m, 2H), 4.33(d, J=11.7Hz, 1H) 4.25(d , J=1.8Hz, 1H), 4.17(d, J=11.7Hz, 1H), 3.04(d, J=6.3Hz, 1H), 2.94(d, J=7.8Hz, 1H), 2.25(s, 3H ), 2.12(s, 3H), 2.08(s, 3H), 2.06(m, 1H), 2.04(m, 1H), 1.87(d, J=2.4Hz, 2H), 1.85(d, J=3.6Hz , 2H), 1.54(d, J=3.9Hz, 1H), 1.36(d, J=2Hz, 2H), 1.18(s, 3H), 1.08(s, 3H).
Embodiment 3
[0045]Example 3.7-((2-((4H-imidazol-2-yl)amino)-1-oxopropan-2-yl)amino)-12-(2-(pyridin-2-yl)amino)-14 Preparation of -deoxy-(E)-15-(4-(dimethylamino)benzylidene)-8,17-epoxyandrographolide: Reagent 7-((4H-imidazol-2-yl) Amino)-1-oxopropan-2-yl)amino-8-oxiranyl-12-(pyridin-2-yl)amino-14-deoxyandrographolide and 2-(4-(dimethylamino ) phenyl)-2-oxoacetic acid, using general reaction method C to obtain the target compound; 1 HNMR: δ7.65(s, 1H), 7.74(m, 2H), 7.58(d, J=9.0Hz, 2H), 7.04(m, 3H), 6.31(m, 1H), 6.06(d, J= 15.6Hz, 1H), 5.80(s, 1H), 5.02(s, 1H), 4.85(d, J=1.8Hz, 2H), 4.15(m, 1H), 3.85(m, 1H), 3.74(m, 1H), 3.33(m, 2H), 3.31(m, 1H), 2.98(s, 6H), 2.90(m, 1H), 2.63(d, J=3.0Hz, 1H), 2.16(d, J=10.2 Hz, 1H), 1.74(m, 3H), 1.58(m, 2H), 1.52(dd, J=3.6Hz, 4.2Hz, 1H), 1.49(m, 2H), 1.32(m, 2H), 1.28( s, 3H), 1.08 (s, 3H), 1.07 (m, 2H), 0.89 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com