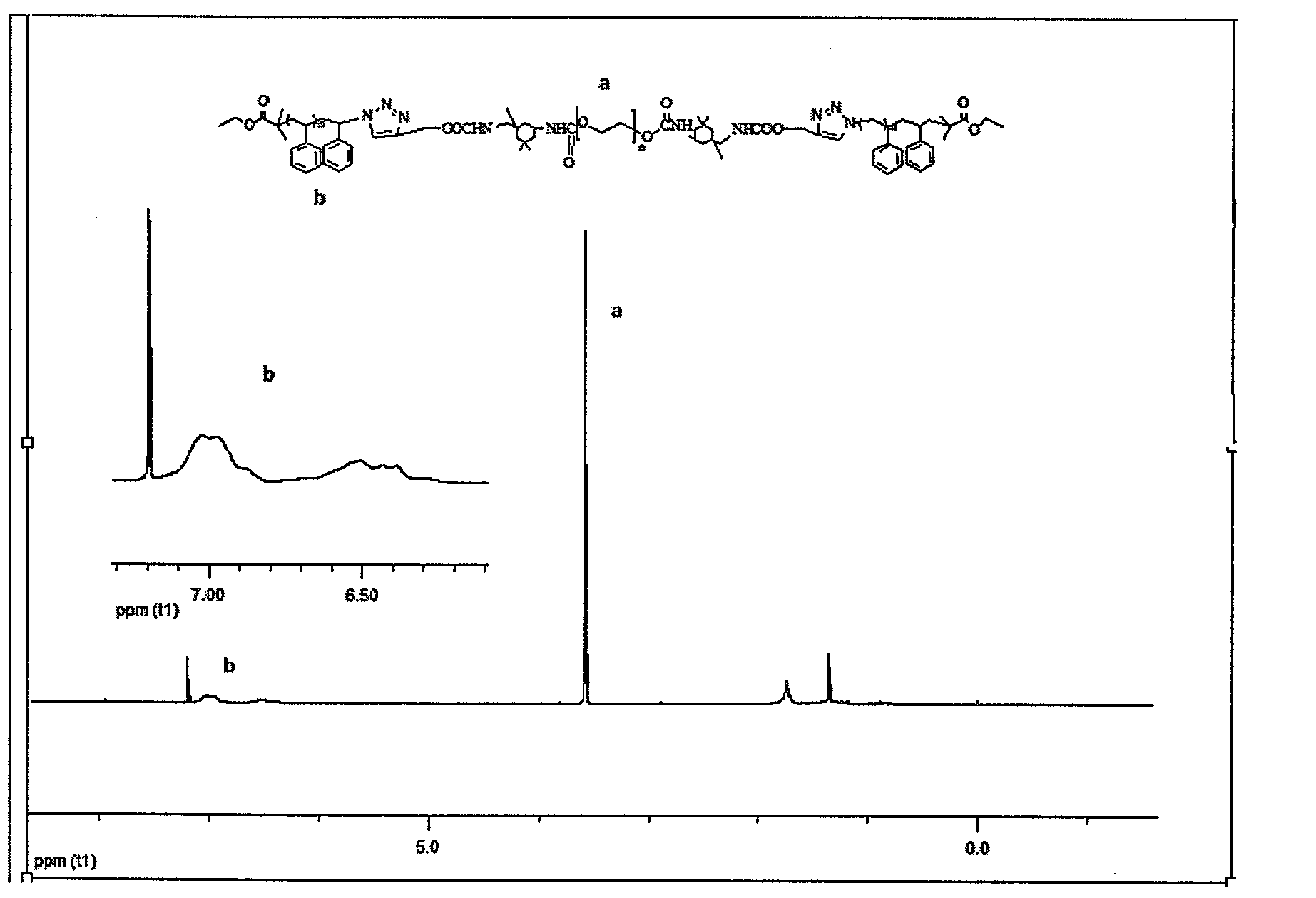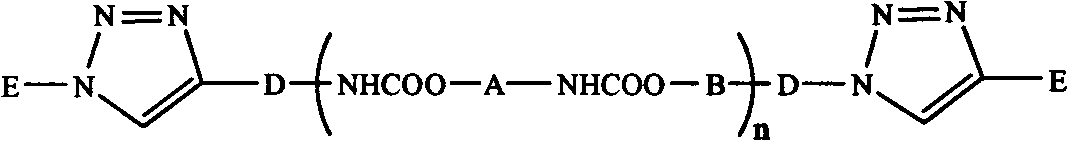Synthetic method of triblock polymer
A polymer and tri-block technology, applied in the field of polymer material synthesis and preparation, can solve the problems of poor controllability of molecular structure, wide molecular weight distribution, harsh reaction conditions, etc., to achieve diversified molecular structure design, mild reaction conditions, The effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
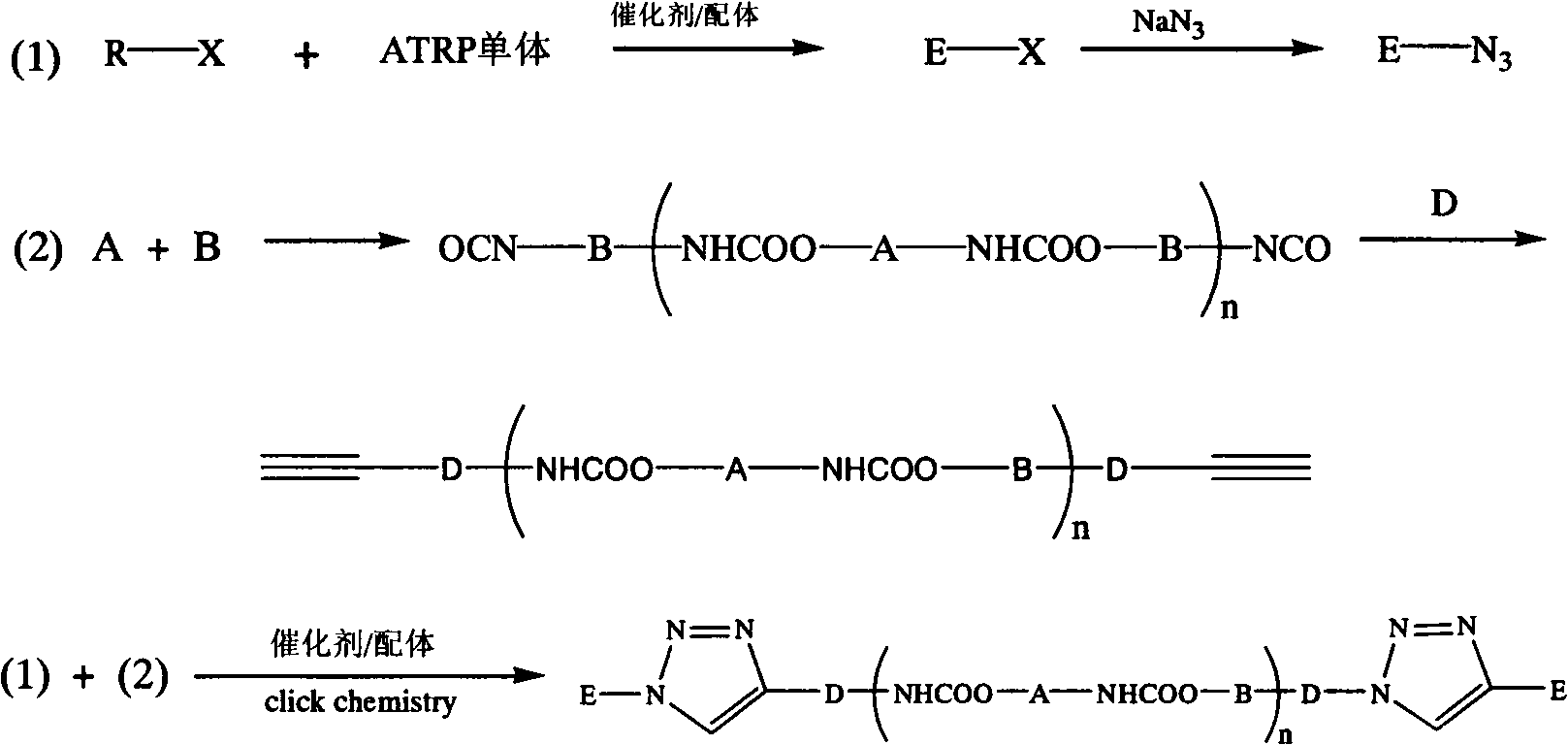
[0035] (1) Synthesis of PS-Br. Put a stirring bar in a clean Schlenk bottle, and add toluene, styrene, PMDETA, and cuprous bromide sequentially according to the molar ratio of 108:192:1:1. After the reaction system was cooled by liquid nitrogen for three times-vacuumizing-nitrogen filling-thawing cycles, ethyl α-bromoisobutyrate (EBiB) was added with a syringe, and the molar ratio of styrene to ethyl α-bromoisobutyrate was 192:1, sealed under a nitrogen atmosphere, reacted at 90°C for 2 hours, cooled with cold water and added to liquid nitrogen to terminate the reaction. After dilution with tetrahydrofuran, neutral alumina column chromatography was used to remove the colored catalyst, and tetrahydrofuran was concentrated by rotary evaporator, and precipitated in a large amount of cold methanol. The dissolution / precipitation operation was repeated three times to obtain a white powder product which was dried in a vacuum oven at 50° C. for 12 hours.
[0036]1 H NMR (CDCl 3 )d ...
Embodiment 2
[0045] (1) Synthesis of PMMA-Br. Put a stirring bar in a clean Schlenk bottle, and add toluene, MMA, PMDETA, and cuprous bromide sequentially according to the molar ratio of 200:200:2:1. After the reaction system was cooled by liquid nitrogen three times-vacuumizing-nitrogen filling-thawing cycle, α-bromoisobutyrate ethyl was added with a syringe, and the molar ratio of MMA to α-bromoisobutyrate was 200:1. Seal it under a nitrogen atmosphere, react at 70°C for 6h, and add liquid nitrogen to terminate the reaction after cooling down with cold water. Use ethanol as precipitant, filter and dissolve in tetrahydrofuran, go through neutral alumina column chromatography to remove colored catalyst, use rotary evaporator to evaporate THF to dryness, and obtain white powdery product and place it in a vacuum oven for drying.
[0046] (2)PMMA-N 3 The synthetic method is with embodiment 1.
[0047] (3) Add polypropylene glycol 2000 to a three-necked bottle equipped with a stirrer and a ...
Embodiment 3
[0050] (1) adopt p-nitrobenzyl bromoisobutyrate except initiator, catalyzer adopts cuprous bromide, ligand adopts bipyridine, initiator: catalyst: ligand: St=1: 1: 2: 200, toluene and The volume ratio of St is 1: 1, the reaction temperature is 110°C, and the reaction time is 4h, the others are the same as step (1) in Example 1.
[0051] (2) Except that the molar ratio of PS-Br and sodium azide is 1:10, and DMF is 20 wt%, other steps are the same as step (2) in Example 1.
[0052] (3), (4) are all with step (3), (4) in embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com