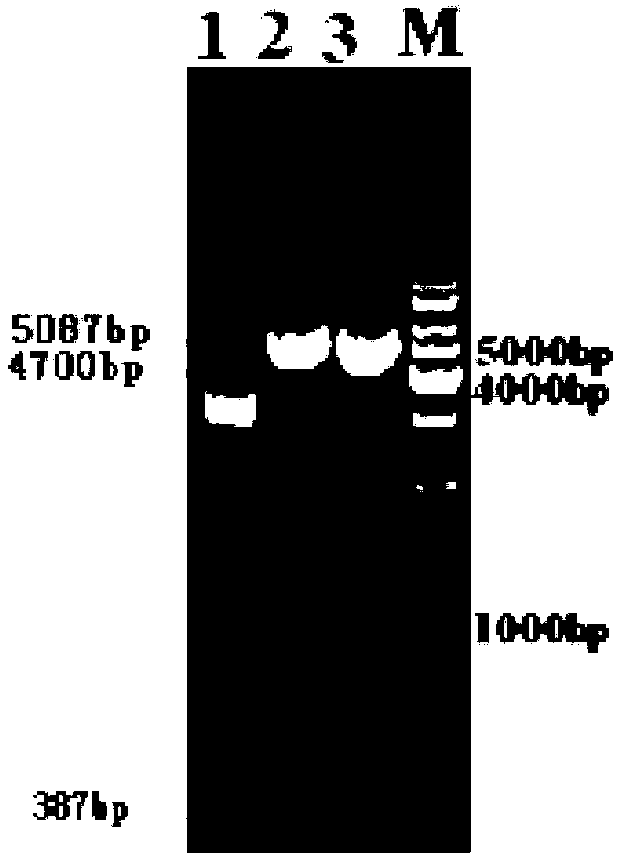Human and mammal cell attachment expression vector and application thereof
An expression vector and mammalian technology, applied in the field of genetic engineering, can solve problems such as diseases and insertion mutations, and achieve high-efficiency expression and good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Construction of Human and Mammalian Cell Attachment Expression Vectors
[0024] 1.1 Materials and methods
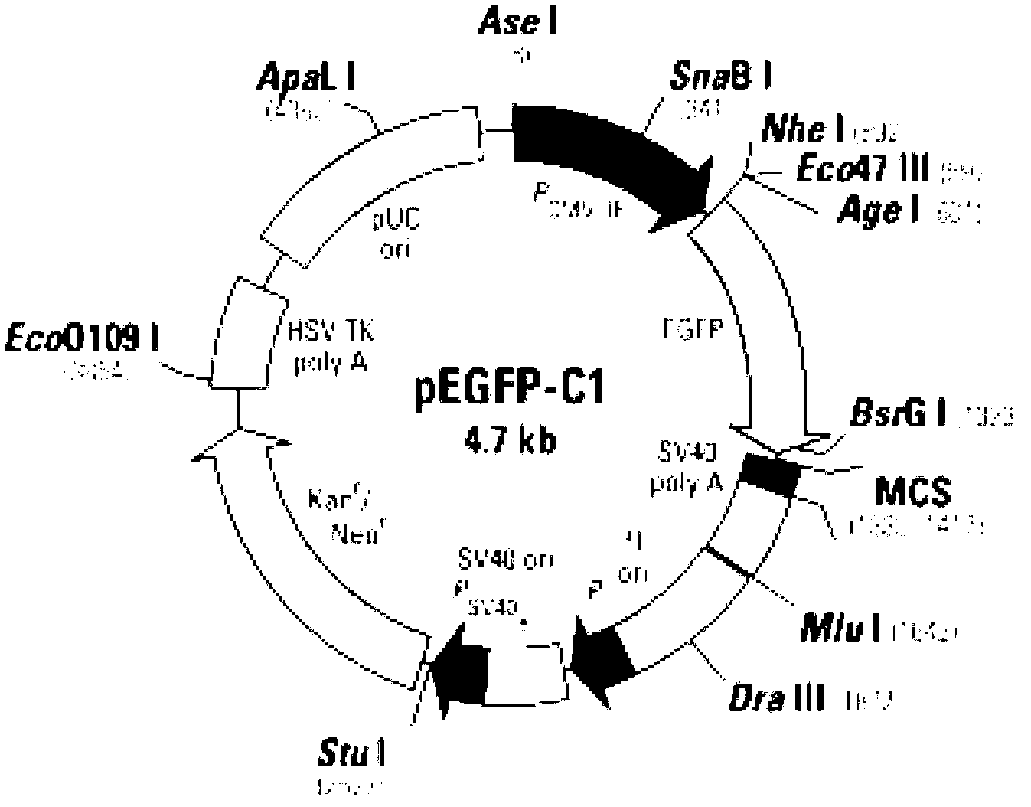
[0025] 1.1.1 pEGFP-C1 vector ( figure 1 ) is an expression vector for human and mammalian cells, which contains the reporter gene EGFP, purchased from Clontech Corporation of the United States.
[0026] Chinese hamster ovary (CHO) cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0027] 1.1.2 Synthesis of MAR sequence
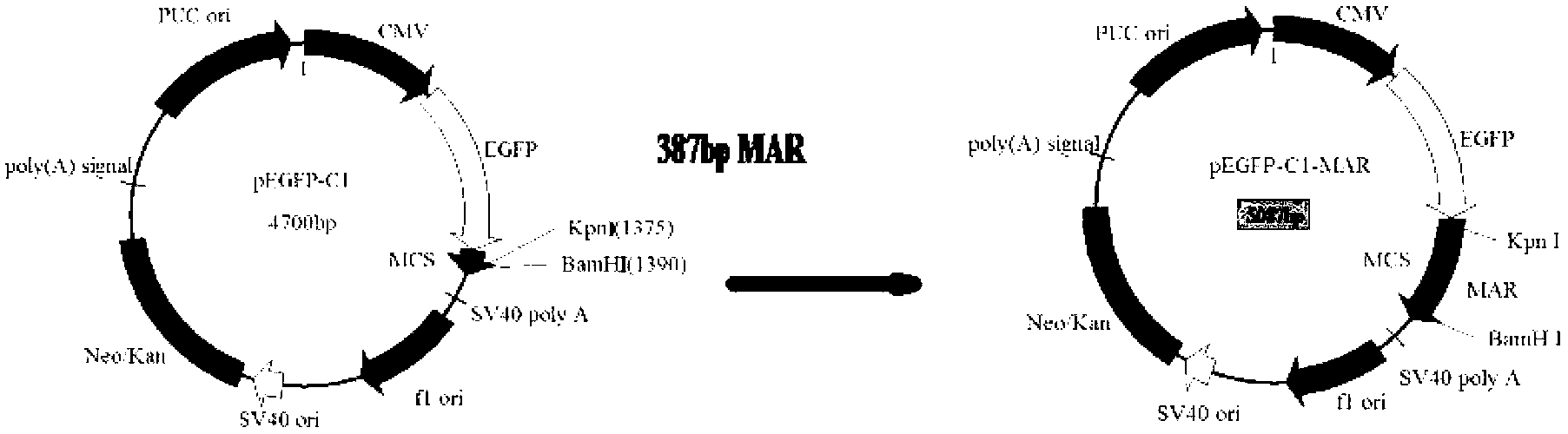
[0028] The 2200bp human β-interferon MAR sequence (GenBank: M83137.1) and the characteristic elements (such as AT-rich sequence) containing the 2150bp MAR sequence (GenBank: L22754.1) of human β-globin were cut and spliced into 387bp , artificially synthesized a short MAR sequence as shown in Seq ID No.1.
[0029] 1.1.3 Enzyme digestion reaction
[0030] Use KpnI and BamHI to double-digest MAR characteristic sequence and pEGFP-C1 vector, the enzyme digestion system is as follows: plasmid 25μl (1...
Embodiment 2
[0036] The screening of embodiment 2 stable monoclonal cell lines
[0037] 1.1 The endotoxin-free pEGFP-C1-MAR plasmid was transfected into CHO cells by Suohua-sofast cationic polymer transfection method, and the CHO cells were cultured in G418 DMEM complete medium with a final concentration of 800 μg / ml.
[0038] 1.2 After positive clones were formed in the CHO cell transfection positive group, and all the cells in the control wells died, they were transferred to DMEM complete medium containing 400 μg / ml G418 for culture.
[0039] 1.3 When the CHO cells reach 70-90%, collect the cells, count them by hemocytometer, dilute with serum-free DMEM medium until the number of cells is about 1 cell in each well, and then add Appropriate amount of complete medium.
[0040] 1.4 Observe the growth of the monoclonal cells, and maintain the selection pressure with DMEM medium at half the concentration of G418. When the monoclonal cell density reaches 1 / 3 of the inner volume of the well, t...
Embodiment 3
[0042] Embodiment 3 plasmid reduction experiment
[0043] 1.1 Utilize the Hirt lysis method to extract the episomal plasmids in two stable monoclonal cells: add 2ml of Hirt lysis buffer to the collected cells, let stand at room temperature for 20min; add 1 / 4 volume (0.5ml) of 5mol / L NaCl, overnight at 4°C. The next day, centrifuge at 15000rpm for 40min. Take the supernatant, add RNase A (10mg / mL) 125μl in 37℃ water bath for 60min; extract twice with phenol chloroform. Take the supernatant, add 5ml -20°C pre-cooled isopropanol and place at -20°C overnight. The next day, centrifuge at 12000rpm for 30min. After washing twice with absolute ethanol and drying in the air, add 10 μl of elution buffer to dissolve the attached body plasmid.
[0044] 1.2 Under sterile conditions, take 250 μl of freshly prepared competent cells and add 10 μl of episomal plasmids for transformation. During recovery, 800 μl of SOC solution preheated at 37°C was added, placed on a shaker at 37°C at 250 r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com