Nitrogenous troxerutin derivative, preparation method thereof and application
A technology for troxerutin and derivatives, which is applied in the field of medicinal chemistry and achieves the effects of easy post-processing, high regioselectivity and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
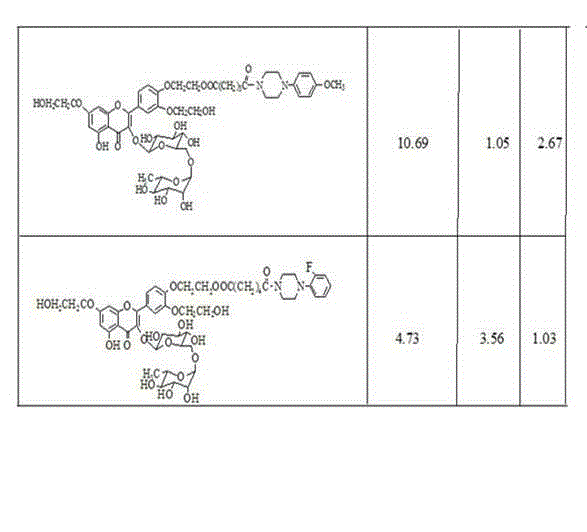
[0024] In a 100 mL Erlenmeyer flask, weigh 560 mg (0.75 mmol) of troxerutin, 582 mg (3 mmol) of divinyl adipate, and 20 mL of pyridine as a solvent, add 600 mg of Bacillus licheniformis alkaline protease, and put Put it into a constant temperature shaker at 50°C and react at a speed of 250 rev·min -1 . After 72 hours of reaction, the enzyme was removed by filtration, and pyridine was distilled off under reduced pressure. Use a mixed solvent of petroleum ether and chloroform with a volume ratio of 3:1 to wash away residual pyridine to obtain a brownish-yellow sticky solid, which is separated and purified by column chromatography, and the eluent is ethyl acetate / methanol / water (15:3.6:0.5 V / V), 403 mg (0.45 mmol) of troxerutin vinyl adipate was obtained as a yellow solid, and the yield was 60%.
[0025] 1 H-NMR (DMSO -d 6 ), δ (ppm): 12.49 (s, 1 H, OH 5 ), 7.84 (s, 1 H, H 2’ ), 7.73 (d, 1H, J=7.2 Hz, H 6’ ), 7.20 (dd, 1 H, J=6.24 Hz, J=14.0 Hz, -OCH=), 7.14 (d, 1 H, J=7....
Embodiment 1-2
[0029] Weigh 560 mg (0.75 mmol) of troxerutin, 756 mg (3 mmol) of divinyl sebacate, and 20 mL of pyridine in a 100 mL Erlenmeyer flask, add 600 mg of Bacillus licheniformis alkaline protease, and put Put it into a constant temperature shaker at 60°C and react at a speed of 250 rev·min -1 . After 120 h of reaction, the enzyme was removed by filtration, and pyridine was distilled off under reduced pressure. Purification as above. 400 mg (0.42 mmol) of troxerutin vinyl sebacoyl was obtained as a yellow solid with a yield of 56%.
[0030] 1 H-NMR (DMSO -d 6 ), δ (ppm): 12.49 (s, 1 H, OH 5 ), 7.84 (s, 1 H, H 2’ ), 7.72 (d, 1H, J=7.0 Hz, H 6’ ), 7.20 (dd, 1 H, J=6.21 Hz, J=14.0 Hz, -OCH=), 7.14 (d, 1 H, J=7.4 Hz, H 5’ ), 6.73 (s, 1 H, H 8 ), 6.38 (s, 1 H, H 6 ), 5.34 (d, 1 H, J=7.3 Hz, H 1’’ ), 4.89 (m, 1 H, OCH=CH 2 ), 4.63 (m, 1 H, OCH=CH 2 ), 4.39 (m, 3 H, 2 H of A acylated, 1 H of B acylated), 4.31 (m, 1 H, H 1’’’ ), 4.26 (m, 1 H, H of B acylated), 4.12-4.06 (m, 4...
Embodiment 1-3
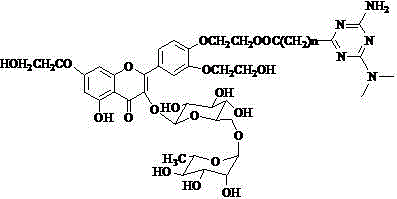
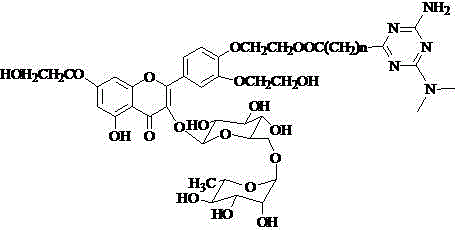
[0034] Add 403 mg (0.45 mmol) of troxerutin vinyl adipate to a 50 mL Erlenmeyer flask, add 576 mg of 1-n-propylpiperazine according to a molar ratio of 1:10, add 25 mL of pyridine as a solvent, and add Lipase LS -10 After adding 600 mg of lipase, place it in a constant temperature shaker at 50°C for reaction at a speed of 250 rev·min -1 . After 120 h of reaction, the reaction was terminated. Post-processing is the same as above. The final product 242mg (0.248mmol) of the piperazine-containing troxerutin derivative was obtained as a yellow solid, and the yield was 55%.
[0035] 1 H-NMR (DMSO -d 6 +D 2 O), δ (ppm): 7.85 (s, 1 H, H 2’ ), 7.73 (d, 1 H, J=7.1 Hz, H 6’ ), 7.16 (d, 1 H, J=7.6 Hz, H 5’ ), 6.71 (s, 1 H, H 8 ), 6.36 (s, 1 H, H 6 ), 5.35 (d, 1 H, J=7.2 Hz, H 1’’ ), 4.40 (m, 3 H, 2 H of A acylated, 1 H of B acylated), 4.30 (m, 1 H, H 1’’’ ), 4.27 (m, 1 H, H of B acylated), 4.11-4.07 (m, 4 H, H of A), 3.75 (m, 4 H, H of B), 3.71-3.11 (10 H, H of rhamnoglucos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com