Detection kit for detecting relative expression level of PML-RAR (alpha) fusion gene by use of fluorescent quantitative PCR (polymerase chain reaction) technology
A detection kit and technology for relative expression, which is applied in the field of detection kits for detecting the relative expression of PML-RARα fusion gene using fluorescent quantitative PCR technology, can solve the problems of high cost and low specificity, and achieve simple operation and high results. Facilitate and improve experimental efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The kit of the present invention includes: it includes the following materials in ratio of parts: erythrocyte lysate (50 parts, 50ml, 10×), TRIzol (50 parts, 50ml), chloroform (50 parts, 30ml), absolute ethanol (50 servings, 40ml), reverse transcription PCR reagent (50 servings, 0.8ml), detection system PCR reaction solution (50 servings, 1.15ml), positive control and negative control;
[0026] Among them, 10× red blood cell lysate (NH4CL 82mg / ml, NAHCO3 8.4mg / ml, EDTA-NA2 3.72mg / ml, DEPC-ddH2O to the prepared volume).
[0027] Reagents for reverse transcription PCR include: 5×RT buffer (120μl), RT Enzyme (25μl), Random Primer (30μl).
[0028] The detection system PCR reaction solution includes: Taq polymerase (1μl), dNTPs (4μl), Tris-HCl buffer (12.5μl), magnesium ion (2μl), and the upstream primers for detecting the target gene are: L-F (0.8μl) or S-F ( 0.8μl), the downstream primer is L / S-R (0.8μl) (L-R is the same as S-R, so L / S-R can be expressed as L-R or S-R), t...
Embodiment 2
[0039] The operation flow of this method:
[0040] (1) Extract tissue RNA from blood: Add 1ml of erythrocyte lysate to a clean 1.5ml centrifuge tube, take 0.5ml of anticoagulated blood and mix well. Let stand at room temperature for 10 minutes; centrifuge at 5000rpm for 5min, discard the supernatant, and collect the cells at the bottom; add 0.5ml red blood cell lysate again, centrifuge at 5000rpm for 5min, discard the supernatant, and collect the cells at the bottom; add 1ml TRIzol to the cells, and pipette repeatedly until sedimentation Dissolve completely, let stand at room temperature for 5 minutes; add 0.2ml chloroform, shake evenly; centrifuge at 14000rpm 4°C for 10 minutes, absorb the supernatant layer and transfer to another new centrifuge tube; add an equal volume of isopropanol, mix well up and down, stand at room temperature Centrifuge at 14000rpm at 4°C for 10min, discard the supernatant, add 1ml of 75% ethanol, wash the tube wall upside down gently; centrifuge at 1...
Embodiment 3
[0050] Using the detection kit of the present invention to detect clinical specimens
[0051] A total of 80 cases of anticoagulant blood samples from patients with acute promyelocytic leukemia (APL) submitted for inspection were extracted, and genomic RNA was extracted, reagents were prepared and tested according to the method described in Example 2.
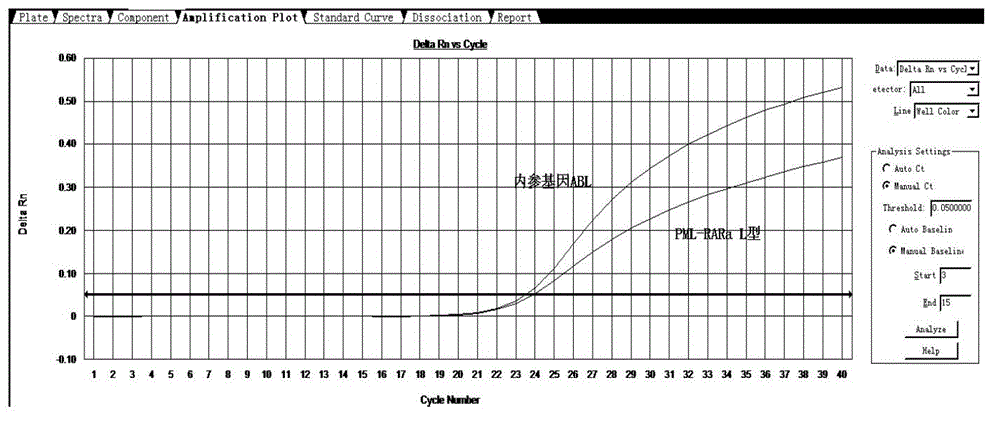
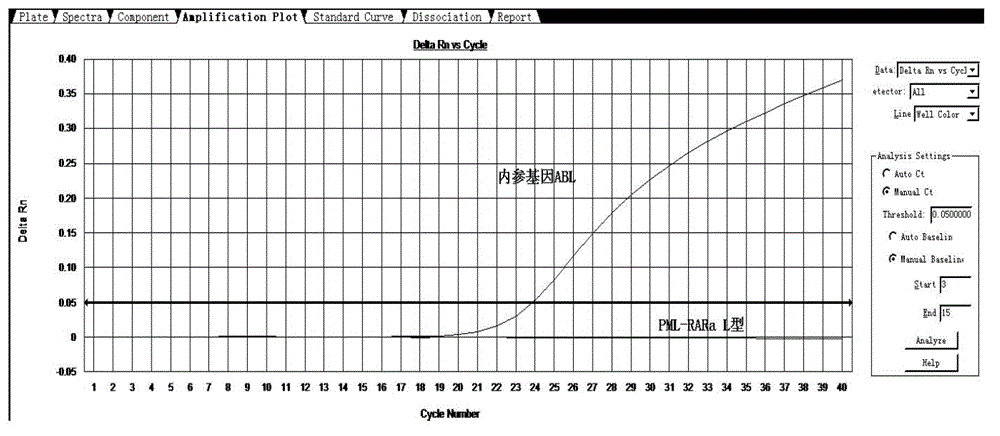
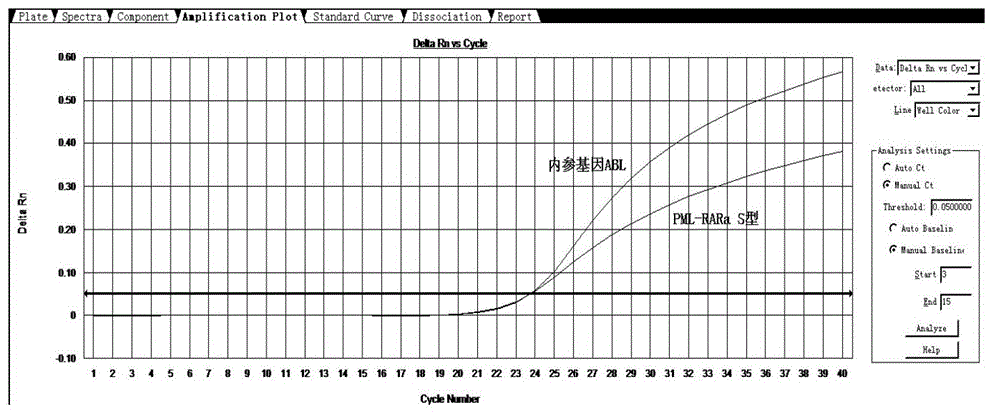
[0052] Take 2 μl of each sample and add it to the detection system PCR reaction solution. At the same time, make a standard curve of positive, negative, blank control, and internal reference gene / target gene. A 96-well fluorescent PCR instrument can detect 38 samples at the same time, each sample is tested twice, a positive control, a negative control and a blank control. The detection time is only 100 minutes.
[0053] The experimental results are compared with the results reported by the special inspection laboratory to determine the accuracy of the sample detection. Some positive results are as follows:
[0054]
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com