Electrodeionization method and electrodeionization apparatus for treating low concentration ionic solution
A technology of electrodeionization and ion solution, which is applied in separation methods, general water supply saving, and separation of dispersed particles. It can solve the problems of increasing ion transfer resistance, increasing membrane stack voltage, hindering ion mass transfer, etc., and achieving adhesion performance. Good, good stability, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
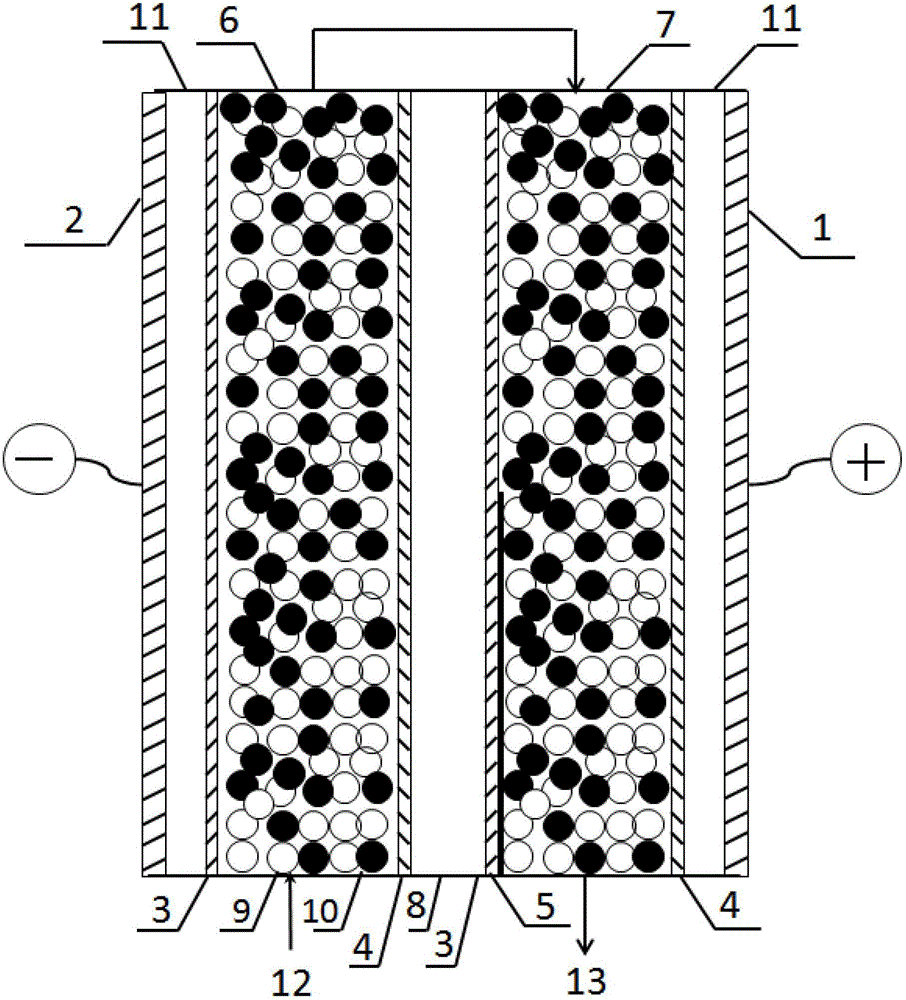
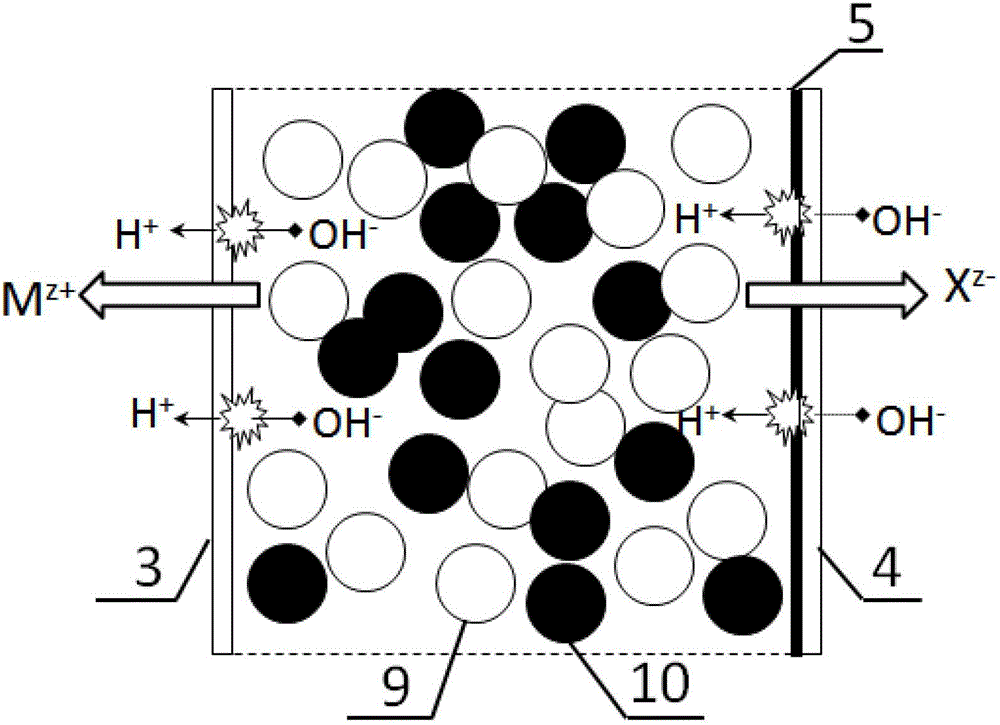
[0032] In this example, the EDI device is one level and two sections, such as figure 1 As shown, there are two light chambers, one concentrated chamber and two pole chambers between the electrodes. A ruthenium dioxide electrode is used as the anode, and a stainless steel electrode is used as the cathode. The size of the separator in the concentrated chamber and the electrode chamber is 100×400×2mm, the size of the separator in the dilute chamber is 100×400×5mm, and the effective area of the membrane is 250cm 2 , using domestic homogeneous anion-cation exchange membrane. The catalyst attached to the cation exchange membrane within the range of the second thin chamber 7 close to 60% of the outlet is chromium hydroxide, and the cation exchange membrane within the range of the first thin chamber 6 remains as it is. Domestic styrene-based strongly acidic cation exchange resin and strongly basic anion exchange resin are used, and the particle size of the resin is 0.5mm. The fil...
Embodiment 2
[0035] The device of embodiment 2, circulation flow process and operating steps etc. are substantially the same as embodiment 1, and difference with embodiment 1 is: the catalyzer attached in the scope close to 60% of outlet on the cation exchange membrane of the second light chamber is nickel hydroxide. Domestic heterogeneous anion and cation exchange membranes are used. After 24 hours of operation, the concentration of copper ions in the effluent of the electrodeionization chamber is lower than 0.22mg / L. After 500 hours of operation of the electrodeionization device, the removal capacity is normal and there is no downward trend. After 500 hours, use an atomic force microscope to observe the catalyst layer on the membrane surface, and the uniformity of catalyst adhesion is consistent with the initial one. Nickel hydroxide catalyst is slightly less effective than chromium hydroxide.
Embodiment 3
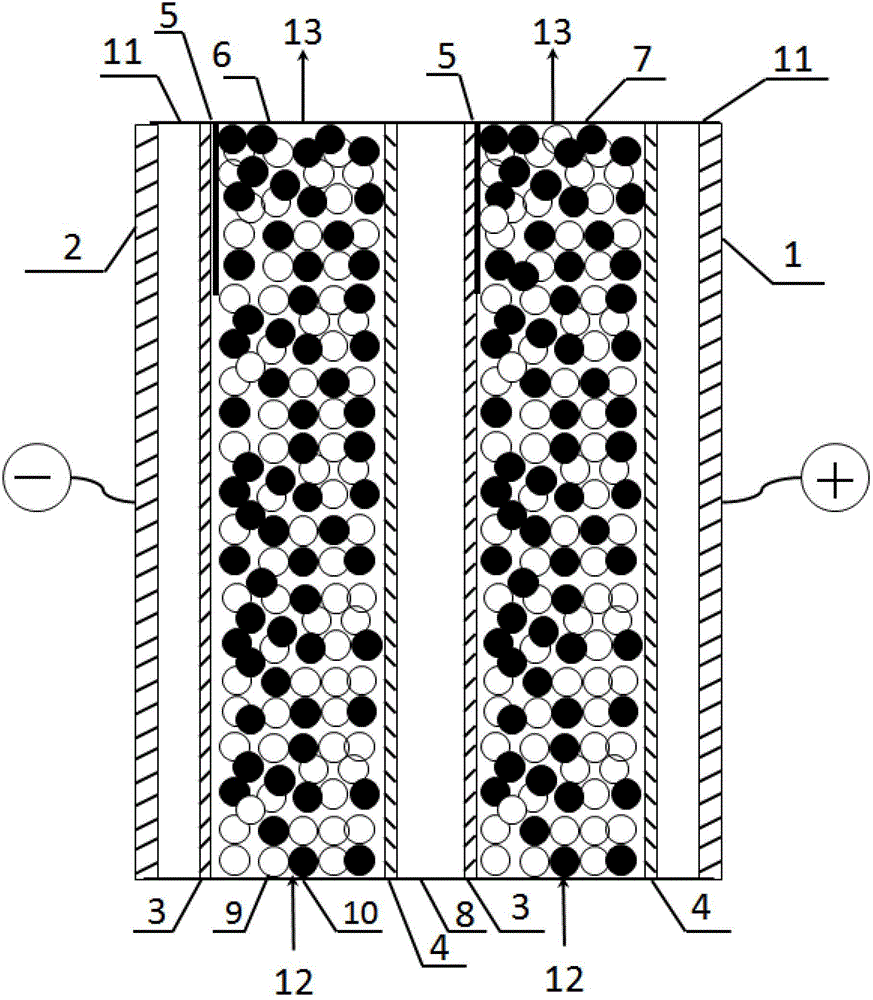
[0037] The device and operating steps of embodiment 3 are basically the same as embodiment 1, and the difference with embodiment 1 is: the EDI device is one stage one section, as figure 2 As shown, the raw water flow rate is 30L / h, and the catalyst attached to the cation exchange membrane within the length range of 35% of the first and second desalination chamber outlets is chromium hydroxide. The filling ratio of the anion and cation exchange resins in the first and second thin chambers was 30% in the area where the catalyst was attached, and 60% in the other areas. After 24 hours of operation, the concentration of copper ions in the effluent of the electrodeionization chamber is lower than 4.0mg / L. After 500 hours of long-term operation, the removal capacity of electrodeionization is normal, and there is no downward trend. After 500 hours of operation of the electrodeionization device, the atomic force microscope was used to observe the catalyst layer on the membrane surfa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com