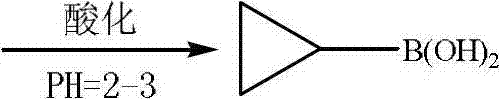
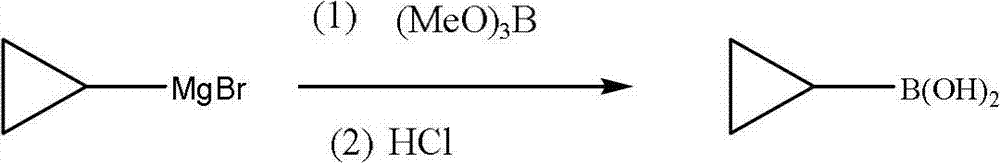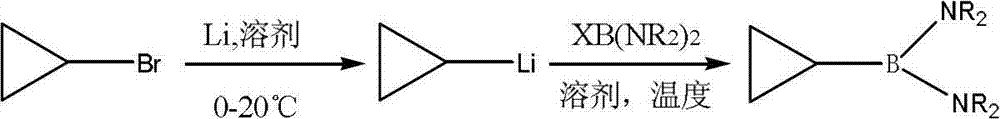Preparation method of cyclopropylboronic acid
A technology of cyclopropylboronic acid and cyclopropylboronic acid ester, which is applied in the field of preparation of cyclopropylboronic acid, can solve the problems of affecting product purity, inconvenient use, and low reaction temperature, so as to facilitate industrial production, reduce production costs, The effect of mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Synthesis of cyclopropyl Grignard reagent:
[0045] In a 10L four-neck flask, add 500ml of anhydrous tetrahydrofuran, 144g (6mol) of magnesium chips, and 50g of cyclopropyl bromide, add a little iodine to initiate, mechanically stir, control the temperature at 40°C, and dropwise add the remaining 555g of cyclopropyl bromide ( A total of 5mol) of THF solution 2.5L. After the dropwise addition, the stirring reaction was continued for half an hour to obtain a tetrahydrofuran solution containing the reaction product cyclopropylmagnesium bromide.
[0046] (2) Synthesis of cyclopropyl borate:
[0047] In a 10L four-neck flask, add 2L of anhydrous tetrahydrofuran, add 675g (6.5mol) of trimethyl borate, cool down to -40°C, add cyclopropylmagnesium bromide tetrahydrofuran solution dropwise, and control the temperature at -35°C~- 45°C, after the dropwise addition, continue to stir for 1h.
[0048] (3) Synthesis of cyclopropylboronic acid sodium salt:
[0049] Add dropwise...
Embodiment 2
[0054] (1) Synthesis of cyclopropyl Grignard reagent:
[0055] In a 10L four-neck flask, add 500ml of anhydrous methyl tert-butyl ether, 180g (7.5mol) of magnesium chips, and 50g of cyclopropyl bromide, add a little iodine to trigger, stir mechanically, control the temperature at 50°C, and add the remaining Cyclopropyl bromide 555g (total 5mol) in anhydrous methyl tert-butyl ether solution 2.5L. After the dropwise addition, the stirring reaction was continued for half an hour to obtain an anhydrous methyl tert-butyl ether solution containing the reaction product cyclopropylmagnesium bromide.
[0056] (2) Synthesis of cyclopropyl borate:
[0057] In a 10L four-neck flask, add 3L of anhydrous methyl tert-butyl ether, add 1095g (7.5) mol of triethyl borate, cool down to -60°C, add cyclopropylmagnesium bromide anhydrous methyl tert-butyl dropwise Base ether solution, control the temperature at -60°C, after the dropwise addition, continue to stir for 1h.
[0058] (3) Synthesis o...
Embodiment 3
[0064] (1) Synthesis of cyclopropyl Grignard reagent:
[0065] In a 10L four-neck flask, add 200ml of anhydrous dipropyl ether, 132g (1.1mol) of magnesium chips, and 50g of cyclopropyl bromide, add a little iodine to initiate, stir mechanically, control the temperature at 40°C, and add the remaining cyclopropyl bromide dropwise. Bromine 555g (total 5mol) in anhydrous dipropyl ether solution 2.5L. After the dropwise addition, the stirring reaction was continued for half an hour to obtain an anhydrous dipropyl ether solution containing the reaction product cyclopropylmagnesium bromide.
[0066] (2) Synthesis of cyclopropyl borate:
[0067] In a 10L four-necked bottle, add 4L of anhydrous dipropyl ether, add 520g (5mol) of trimethyl borate, cool down to -35°C, add dropwise anhydrous dipropyl ether solution of cyclopropylmagnesium bromide, and control the temperature at -30°C~-40°C, after the dropwise addition, continue to stir for 1h.
[0068] (3) Synthesis of cyclopropylboron...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com