Aspirin pulsed release pellets, its preparation and preparation method thereof
A technology of aspirin and pulse release, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as easy hydrolysis, unstable gastrointestinal tract, bleeding, and reduced antithrombotic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Table 1 Embodiment 1 Aspirin pulse release micropellet each layer raw material composition ratio
[0078] category
Proportion
The composition ratio of each layer
[0079] blank core
11.4
\
drug layer
11.4
drug 30, povidone 60, tartaric acid 3, sodium lauryl sulfate 2, crospovidone 5
2.2
Hydroxypropyl Methyl Cellulose 80, Macrogol 10, Talc 10
Regulatory release layer
25
Citric acid 30, enteric material 60, triethyl citrate 5, talcum powder 5
The protective layer
50
Sodium chloride 5, enteric-coated material 70, triethyl citrate 10, glyceryl monostearate 15
[0080] Table 2 is the specific type and dosage of each component in Table 1
[0081]
[0082] Note: The adjusted release layer and protective layer in the table are also listed in the raw material formula of pellets I, II and III
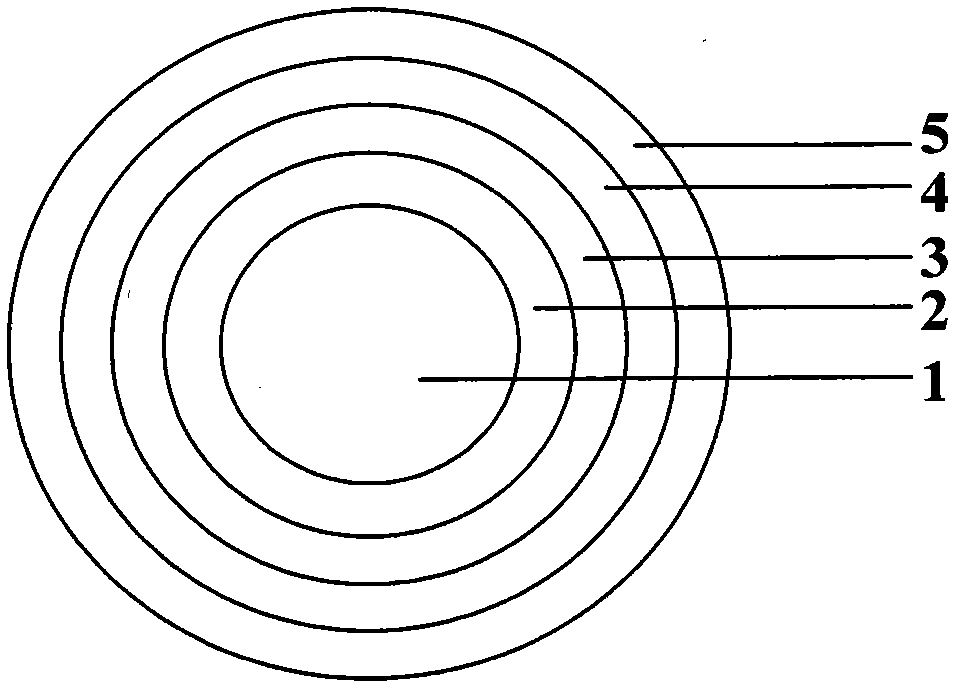
[0083] The structure of the aspirin pulse rel...
Embodiment 2
[0106] Table 3 Embodiment 2 Aspirin pulse release micropellet each layer raw material composition ratio
[0107] category
Proportion
The composition ratio of each layer
blank core
11.4
\
drug layer
11.4
Drug 50, Povidone 40, Tartaric Acid 3, Sodium Lauryl Sulfate 2, Crospovidone 5
2.2
Hydroxypropyl Methyl Cellulose 85, Macrogol 5, Talc 15
Regulatory release layer
25
Citric acid 30, enteric material 60, triethyl citrate 5, talcum powder 5
The protective layer
50
Sodium chloride 0, enteric material 90, triethyl citrate 5, glyceryl monostearate 5
[0108] Table 4 is the specific type and dosage of each component in Table 3
[0109]
[0110]
[0111] Preparation:
[0112] 1. Drug-coated layer: same as in Example 1
[0113] 2. Packet isolation layer: same as embodiment 1
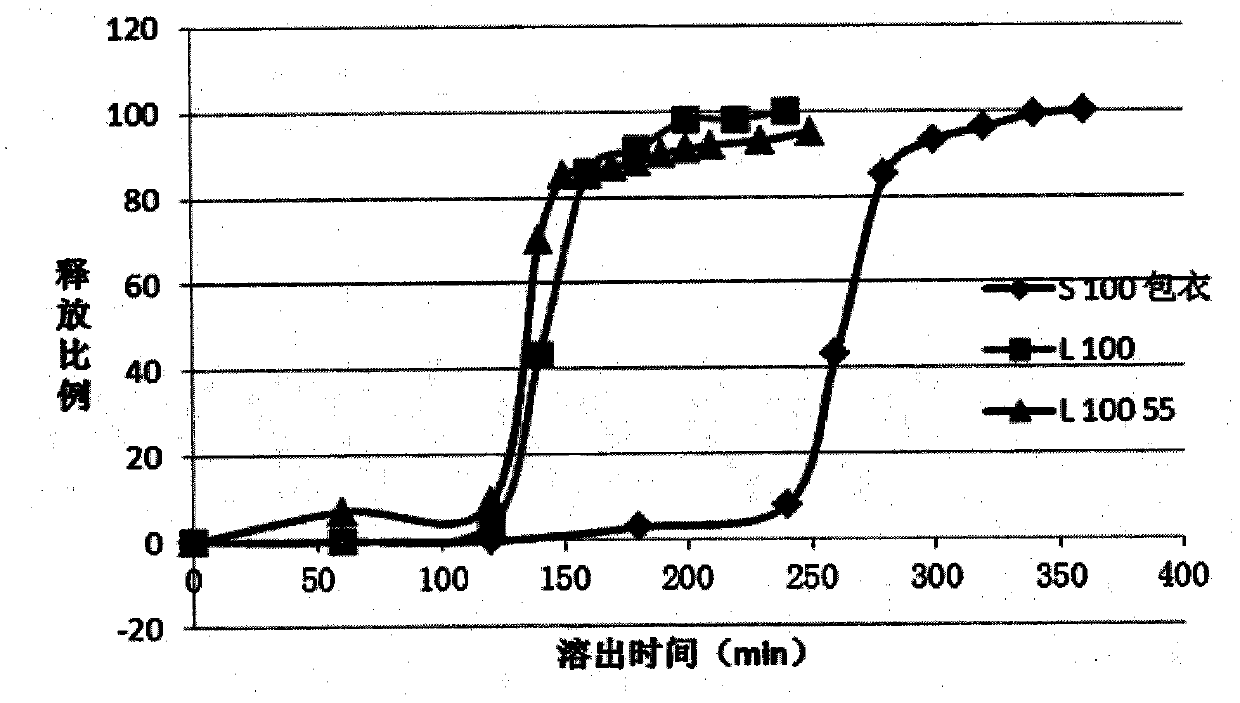
[0114] 3, bag regulating release layer: prepare I, II, III micropill with e...
Embodiment 3
[0119] Table 5 Embodiment 3 aspirin pulse release micropellet each layer raw material composition ratio
[0120] category
Proportion
The composition ratio of each layer
blank core
11.4
\
drug layer
11.4
drug 40, povidone 50, tartaric acid 1, sodium lauryl sulfate 4, crospovidone 5
2.2
Hydroxypropyl Methyl Cellulose 85, Macrogol 5, Talc 15
Regulatory release layer
25
Citric acid 20, enteric material 60, triethyl citrate 5, talcum powder 15
The protective layer
50
Sodium chloride 0, enteric-coated material 80, triethyl citrate 5, glyceryl monostearate 15
[0121] Table 6 is the specific type and dosage of each component in Table 5
[0122]
[0123]
[0124] Preparation:
[0125] 1. Drug-coated layer: same as in Example 1
[0126] 2. Packet isolation layer: same as embodiment 1
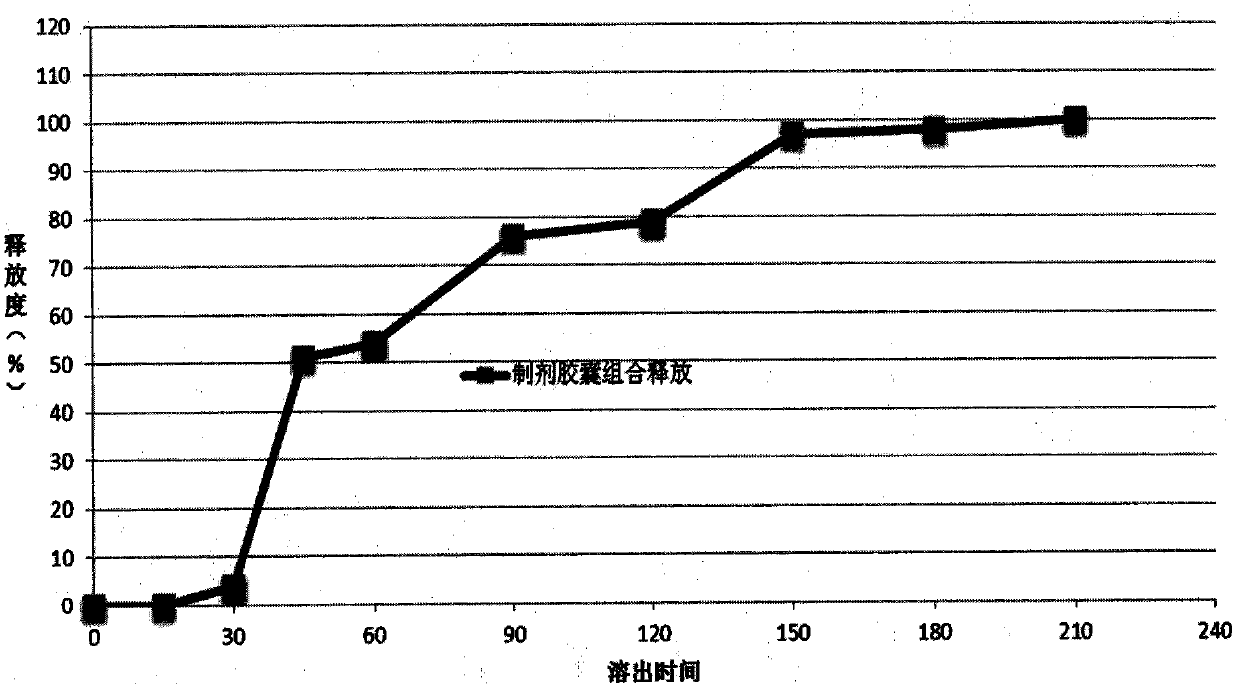
[0127] 3, bag regulating release layer: prepare I, II, III micropill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com