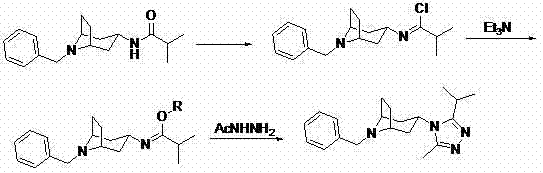
Preparation method of 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo (3.2.1)octane
A -4H-1, azabicycle technology, applied in organic chemistry and other directions, can solve the problems of low product yield and purity, interfere with recrystallization, etc., and achieve the effects of good product purity, complete reaction, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] PCl 5 (1.46g, 7.0mmol) plus CH 2 Cl 2 (22.6mL, 30.0g), cooled in an ice-salt bath, added N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutyramide (2.0g, 7.0mmol), After stirring for half an hour, remove ice water, rise to room temperature and stir for 1.5 h, cool with ice water, add ethanol (1.97 mL, 35.0 mmol) and Et 3 N (1.95mL, 14.0 mmol) mixed solution, after dripping, remove ice water, raise room temperature and stir overnight, then cool in ice water, add NaOH aqueous solution dropwise to pH>9, separate organic layer, and wash water layer with CH 2 Cl 2 (50mlx2) extraction, combined organic phases, and evaporated to dryness under reduced pressure to obtain ethyl N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (2.02g , yield 91.7%).
[0016] Dissolve ethyl N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (1.57g, 5.0mmol) obtained in the previous step in isoamyl alcohol ( 34.9mL, 28.26g), add acetylhydrazide (0.56g, 7.5mmol), heat to reflux for 3h, ...
Embodiment 2
[0018] SOCl 2 (1.66g, 14.0mmol) plus CHCl 3 (27.0mL, 40.0g), cooled in an ice-salt bath, added N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutyramide (2.0g, 7.0mmol), After stirring for half an hour, remove ice water, rise to room temperature and stir for 1 h, cool with ice water, add isopropanol (5.4 mL, 70.0 mmol) and Et 3 N (3.4mL, 24.5mmol) mixed solution, after dripping, remove ice water, raise room temperature and stir overnight, then cool with ice water, add KOH aqueous solution dropwise to pH>9, separate organic layer, and wash water layer with CH 2 Cl 2 (50mlx2) extraction, combined organic phases, and evaporated to dryness under reduced pressure to obtain isopropyl N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (2.12 g, yield 92.2%).
[0019] Dissolve N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (1.64g, 5.0mmol) in ethanol (41.5 mL, 32.8g), add acetylhydrazide (0.74g, 10mmol), heat to reflux for 2h, evaporate the solvent under reduced pressure...
Embodiment 3
[0021] Add oxalyl chloride (2.7g, 21.0mmol) to 1,2-dichloroethane (39.8mL, 50g), cool in an ice-salt bath, add N-(8-benzyl-8-azabicyclo[3.2.1 ]oct-3-yl)isobutyramide (2.0g, 7.0mmol), stirred for half an hour, removed ice water, raised to room temperature and stirred for 2h, cooled with ice water, added dropwise benzyl alcohol (10.9mL, 105.0mmol) and Et 3 N (4.9 mL, 35.0 mmol) mixed solution, drop off, remove ice water, raise room temperature and stir overnight, then cool in ice water, add NaOH aqueous solution dropwise to pH>9, separate organic layer, and wash the water layer with CH 2 Cl 2 (50mlx2) extraction, combined organic phases, and evaporated to dryness under reduced pressure to obtain benzyl N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (2.42g , yield 92.0%).
[0022] Dissolve the benzyl N-(8-benzyl-8-azabicyclo[3.2.1]oct-3-yl)isobutylimidate (1.88g, 5.0mmol) obtained in the previous step in isobutanol ( 51.1mL, 41.36g), add acetylhydrazide (0.93g, 12.5mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com