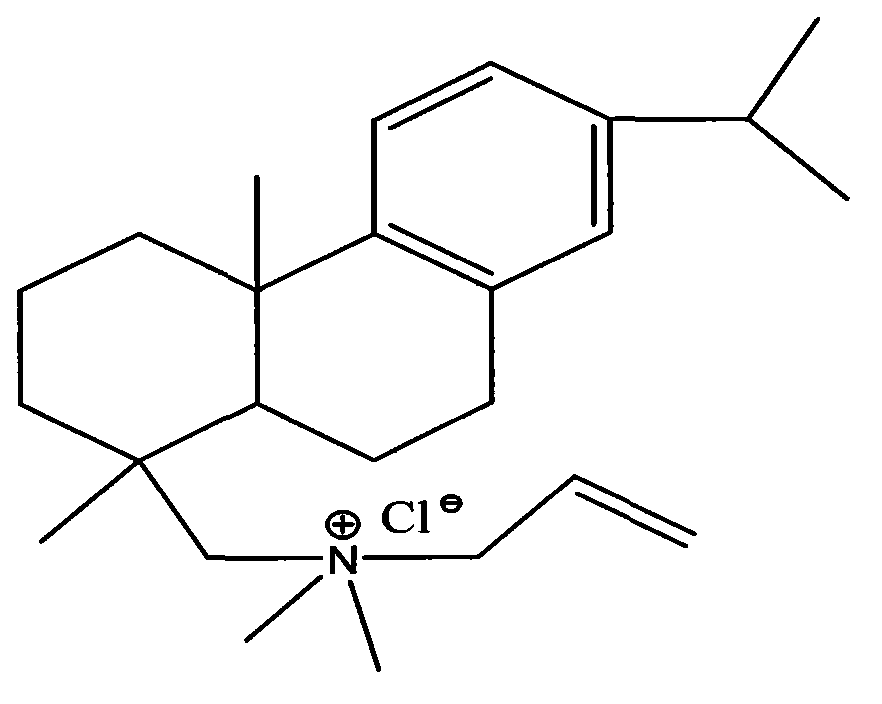
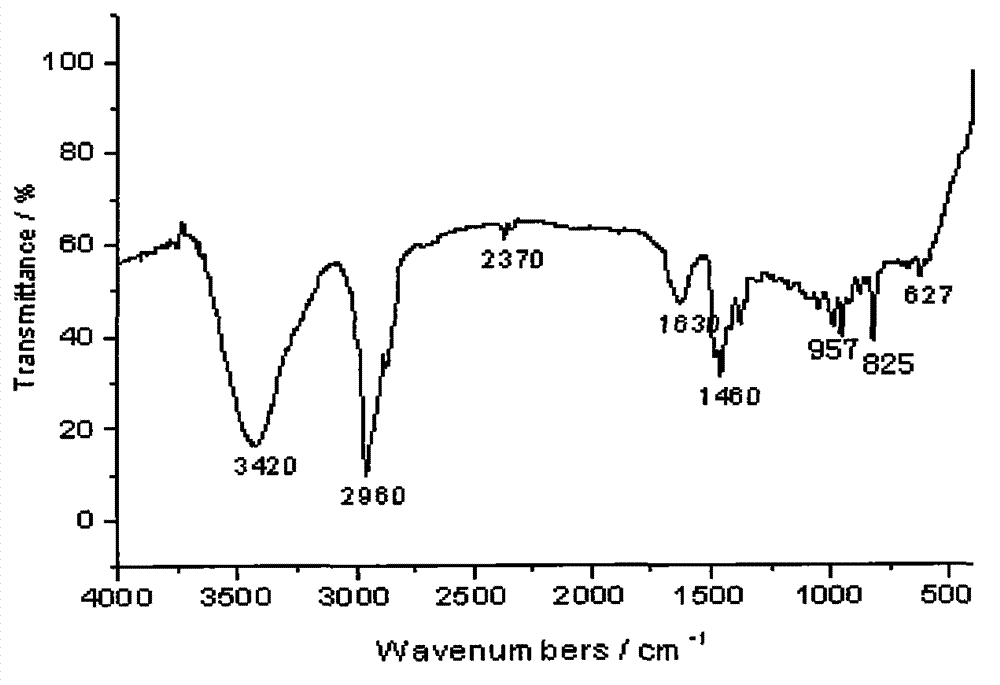
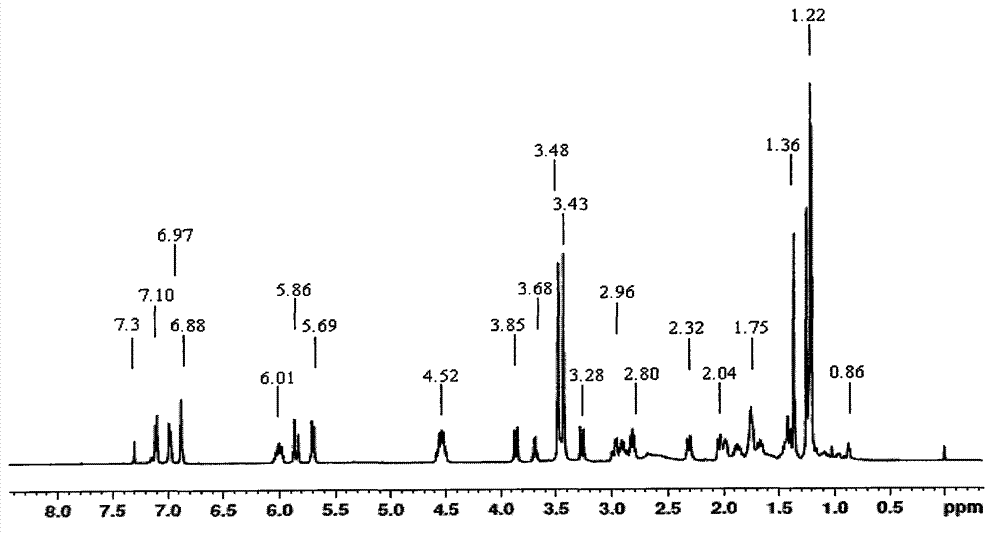
Method for preparing allyldimethyl dehydroabietyl ammonium chloride
A technology for allyl dimethyl dehydroabietyl and dimethyl dehydroabietin amine, which is applied in the field of preparing cationic surfactants containing dehydroabietin amine structure, and can solve the problem of affecting product application performance, decreasing reaction activity, etc. problem, to achieve the effect of excellent antistatic performance, low preparation cost and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 100 grams of dehydroabietic amines are dissolved with 400 grams of composite solvents composed of ethanol and acetonitrile in a mass ratio of 0.01: 7 to form a homogeneous solution, then add 600 grams of formic acid aqueous solution with a mass percentage concentration of 30%, and stir to form The solution of abietamine formate; Add 50 grams of methylation reagents consisting of formaldehyde and paraformaldehyde in a mass ratio of 1.0:5.0 to the solution containing dehydroabietamine formate, and react at 40°C After 60 hours, the mixed material containing N,N-dimethyl dehydroabietamine formate was obtained; the mixed material containing N,N-dimethyl dehydroabietamine formate was neutralized, extracted with toluene, saturated salt Water washing, water washing and liquid separation, normal pressure or reduced pressure distillation to recover toluene and vacuum fractionation to obtain N,N-dimethyl dehydroabietic amine and compound inhibitor according to the mass ratio of 0.1...
Embodiment 2
[0022] 100 grams of dehydroabietic amines are dissolved with 800 grams of ethanol and acetonitrile in a composite solvent of 0.1: 10 by mass ratio to form a homogeneous solution, then add 900 grams of formic acid aqueous solution with a mass percentage concentration of 50%, and stir to form a compound solvent containing dehydrogenated The solution of abietamine formate; Add 100 grams of methylation reagents consisting of formaldehyde and paraformaldehyde in a mass ratio of 7:15 to the solution containing dehydroabietamine formate, and react at 70°C In 36 hours, the mixed material containing N,N-dimethyl dehydroabietamine formate was obtained; the mixed material containing N,N-dimethyl dehydroabietamine formate was neutralized, extracted with toluene, saturated salt Water washing, water washing and liquid separation, normal pressure or reduced pressure distillation to recover toluene and vacuum fractionation to obtain N,N-dimethyl dehydroabietine amine and compound inhibitor acc...
Embodiment 3
[0024]100 grams of dehydroabietic amines are dissolved with 1200 grams of composite solvents composed of ethanol and acetonitrile in a mass ratio of 3: 13 to form a homogeneous solution, then add 900 grams of formic acid aqueous solution with a mass percentage concentration of 70%, and stir to form The solution of abietamine formate; Add 150 grams of methylation reagents consisting of formaldehyde and paraformaldehyde in a mass ratio of 5.0:3 to the solution containing dehydroabietamine formate, and react at 60°C In 48 hours, the mixed material containing N,N-dimethyl dehydroabietamine formate was obtained; the mixed material containing N,N-dimethyl dehydroabietamine formate was neutralized, extracted with toluene, saturated salt Water washing, water washing and liquid separation, normal pressure or reduced pressure distillation to recover toluene and vacuum fractionation to obtain N,N-dimethyl dehydroabietine amine and composite inhibitor in mass ratio 0.1:0.01 and then use ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com