Synthesis technique of brominated butyl rubber
A brominated butyl rubber and synthesis process technology, applied in the field of brominated butyl rubber synthesis process, can solve problems such as difficult product quality, high energy consumption, difficult control, etc., to reduce the probability of transposition side reactions, energy The effect of low consumption and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
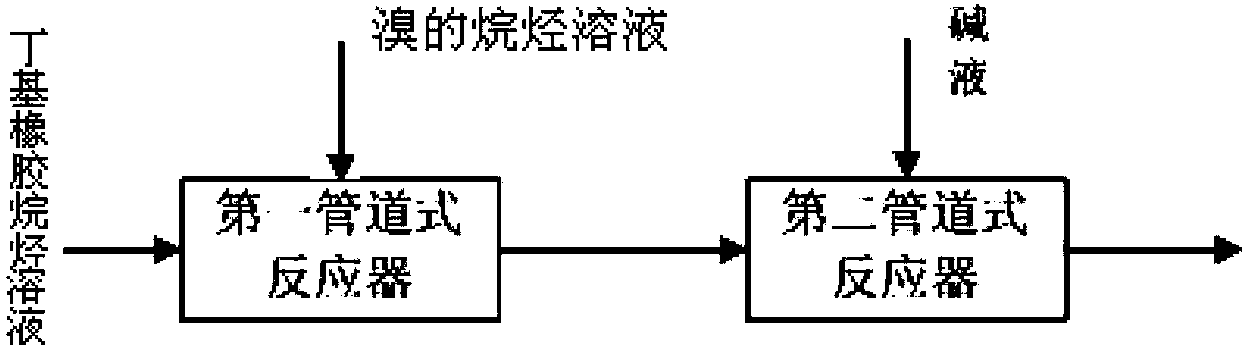
[0018] according to figure 1 As shown in the flow chart, the concentration of butyl rubber is 25wt% n-hexane solution, Br 2 Concentration is 20wt% n-hexane solution, lye is 10wt% sodium hydroxide aqueous solution as raw material, wherein the unsaturation degree of butyl rubber is 1.9%, Br 2 The molar ratio of the double bond in butyl rubber is 0.7, NaOH and Br 2 The molar ratio is 1:1. The temperature of each feed is 30oC; The flow velocity in the follow-up pipeline of the first pipeline reactor is 0.3m / s, and the residence time is 60 seconds; The flow velocity in the follow-up pipeline of the second pipeline reactor is 0.3m / s, and the residence time is 300 seconds. The product bromobutyl rubber has a bromination degree of 2%, a secondary bromine content of 70%, a degree of unsaturation of 0.5%, a rubber number average molecular weight of 180,000 Daltons, and a molecular weight distribution PDI of 2.5.
Embodiment 2
[0020] according to figure 1 The flow chart shown, with butyl rubber concentration as 5wt% n-heptane solution, Br 2 The n-heptane solution with a concentration of 10wt% and the sodium hydroxide aqueous solution with a 2wt% lye are used as raw materials. The unsaturation of butyl rubber is 1.2%, and the Br 2 The molar ratio to the double bond in butyl rubber is 1.1, NaOH and Br 2 The molar ratio is 2:1. The temperature of each feed is 20oC; the flow velocity in the follow-up pipeline of the first pipeline reactor is 1m / s, and the residence time is 10 seconds; the flow velocity in the follow-up pipeline of the second pipeline reactor is 1m / s, and the residence time is 60 seconds . The product bromobutyl rubber has a degree of bromination of 0.8%, a secondary bromine content of 99%, a degree of unsaturation of 0.5%, a rubber number average molecular weight of 230,000 Daltons, and a molecular weight distribution PDI of 1.9.
Embodiment 3
[0022] according to figure 1 As shown in the flowchart, the concentration of butyl rubber is 5wt% n-octane solution, Br 2 The n-octane solution with a concentration of 2wt% and the aqueous sodium hydroxide solution with a 0.1wt% lye are used as raw materials. The degree of unsaturation of butyl rubber is 3.0%. Br 2 The molar ratio to the double bond in butyl rubber is 2.0, NaOH and Br 2 The molar ratio is 1.5:1. The temperature of each feed is 60oC; the flow velocity in the follow-up pipeline of the first pipeline reactor is 2m / s, and the residence time is 6 seconds; the flow velocity in the follow-up pipeline of the second pipeline reactor is 2m / s, and the residence time is 20 seconds . The degree of bromination of the product bromobutyl rubber is 1.2%, the secondary bromine content is 99%, the degree of unsaturation is 1.2%, the number average molecular weight of the rubber is 220,000 Daltons, and the molecular weight distribution PDI is 2.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com