Reagent kit for distinguishing and detecting dengue virus/yellow fever virus/west nile virus/chikungunya virus
A technology for chikungunya virus and West Nile virus, which can be used in the determination/inspection of microorganisms, fluorescence/phosphorescence, resistance to vector-borne diseases, etc., and can solve the problems of easy contamination, low sensitivity, and immune cross-reaction cycle Achieve the effect of reducing pollution, high throughput and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
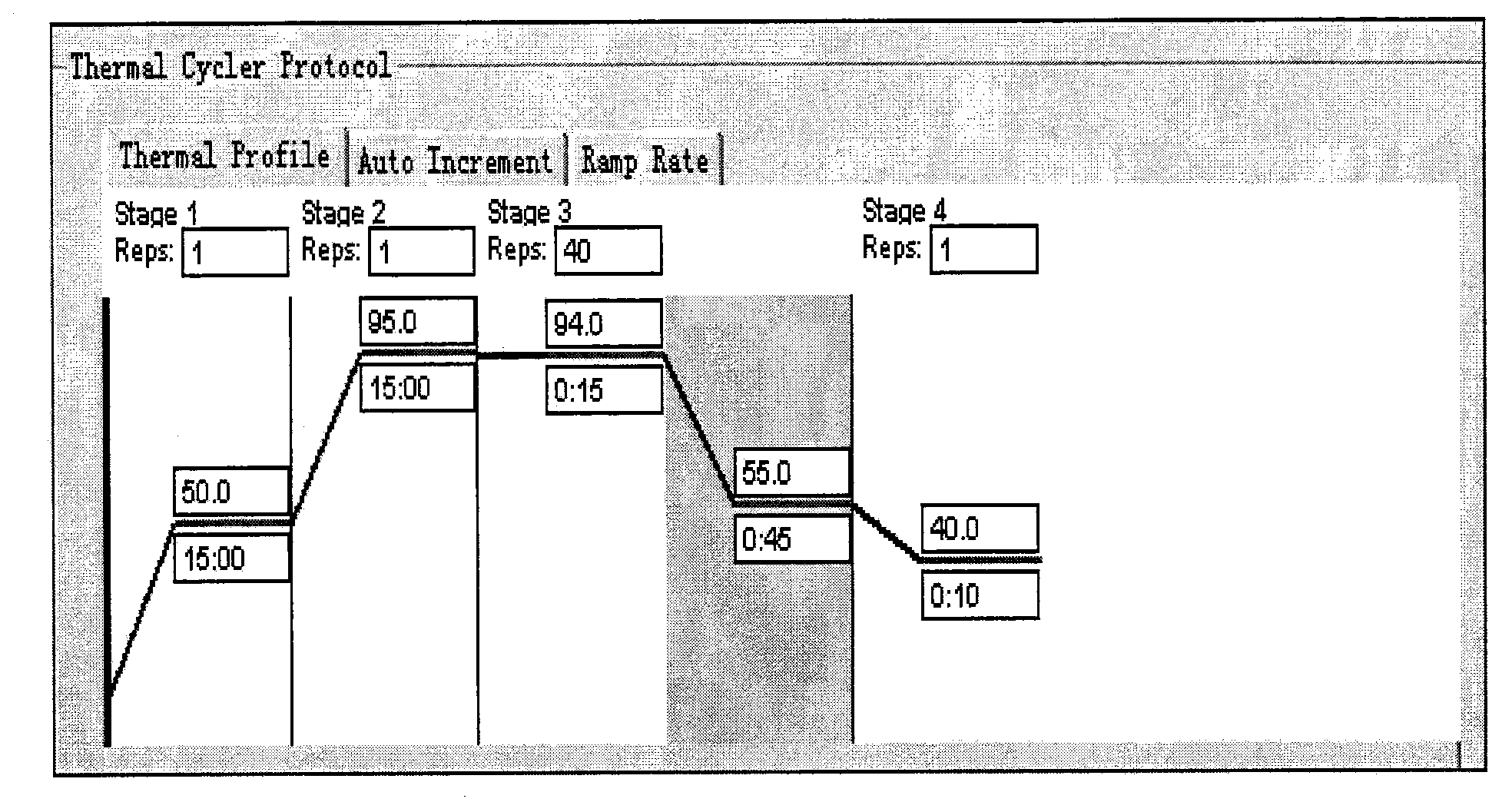
[0030] Example 1 Dengue virus / yellow fever virus / West Nile virus / Chikungunya virus identification and detection kit and its use Tube, RT-PCR reaction solution (250μl / tube), RT-PCR enzyme system (75μl / tube) 1 tube, positive quality control (200μl / tube) 1 tube, negative quality control (200μl / tube) 1 tube , DEPC H 2 O (2000μl / tube) 1 tube.
[0031] 2. Collection, preservation and transportation of specimens
[0032] 2.1 Specimen types: serum, mosquito samples.
[0033] 2.2 Specimen collection:
[0034] 2.2.1 Serum: Aseptically collect 3-5ml of venous blood within 5 days after the onset of the disease, let it stand at room temperature for 30 minutes to solidify, then centrifuge at 1500-2000rpm for 10min to remove fibrin and red blood cells, and collect serum in 2ml of sterile screw-top plastic tube.
[0035] 2.2.2 Mosquitoes: collect mosquito samples according to actual work needs. Put the collected mosquitoes directly into the -20°C refrigerator to freeze to death, take th...
Embodiment 2
[0047] Example 2 Application of dengue virus / yellow fever virus / West Nile virus / chikungunya virus differential detection kit to detect clinical samples
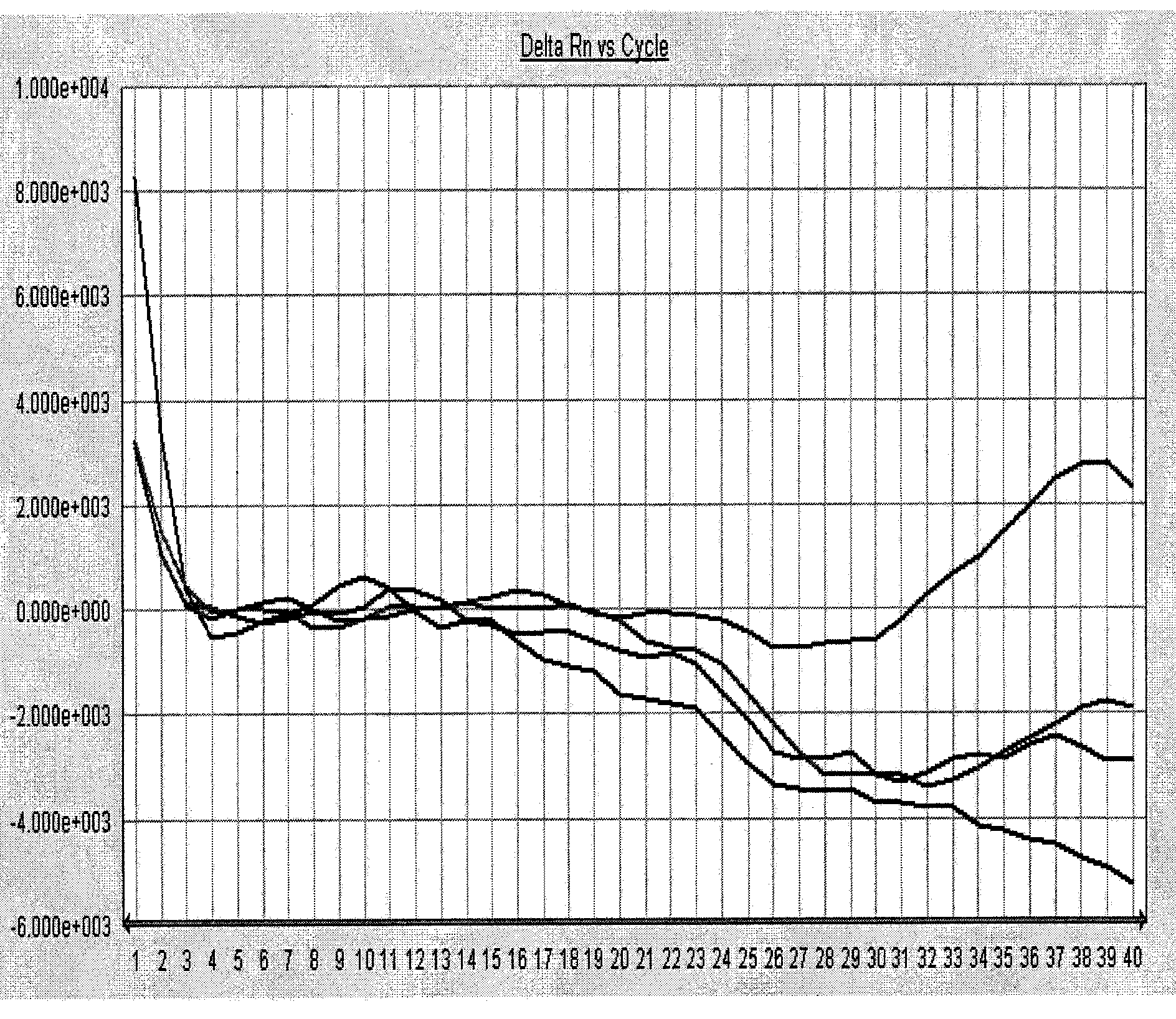
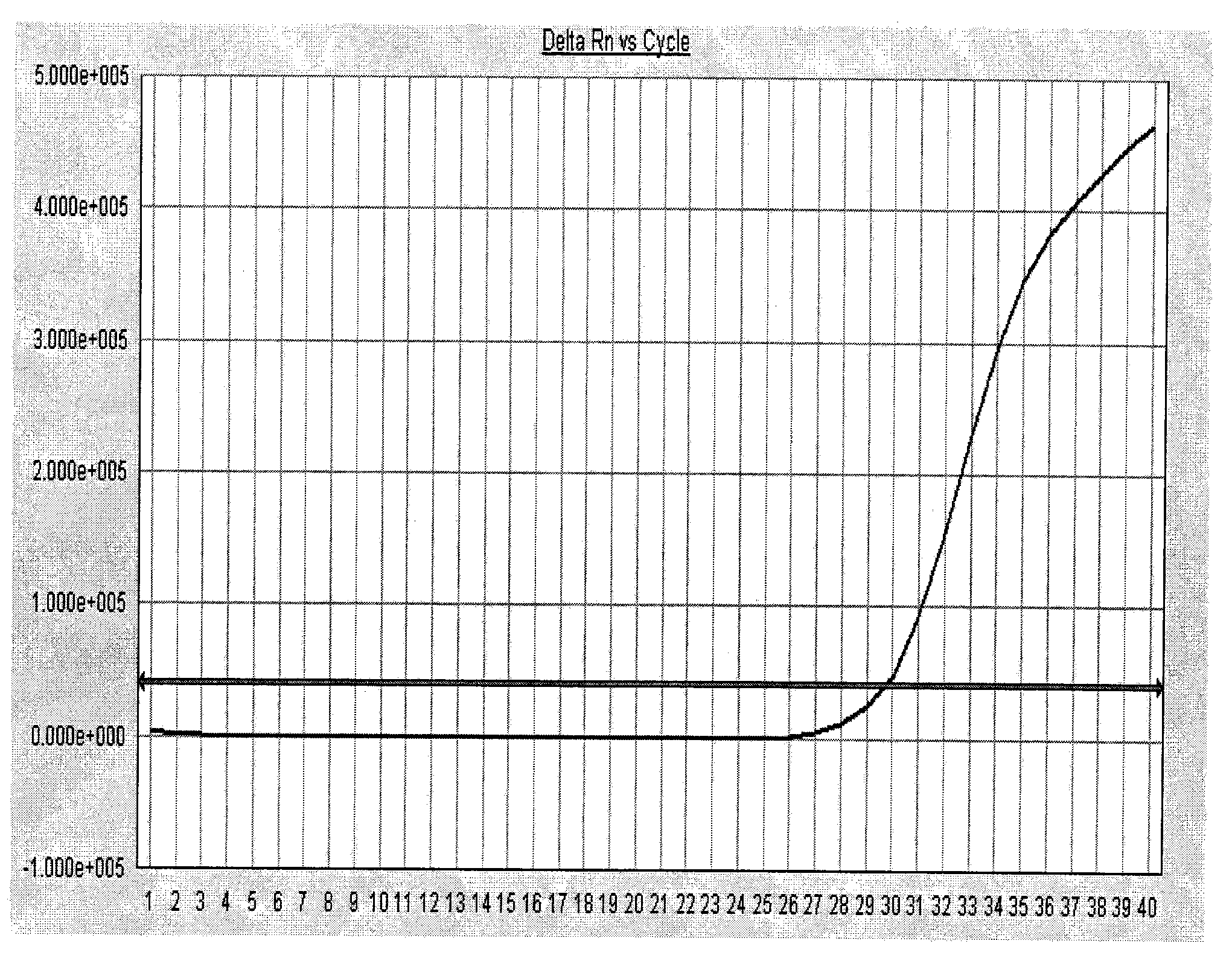
[0048] The positive serum samples of dengue virus type I, dengue virus type II, dengue virus type III, dengue virus type IV, yellow fever virus, West Nile virus, and chikungunya virus were selected and identified by the virus culture method. One case, and one case of serum samples each positive for Japanese encephalitis virus, fever with thrombocytopenia syndrome bunya virus, Hantaan virus, and Seoul virus identified by virus culture methods were used as specific samples, and all samples were tested for nucleic acid. The steps of extraction, PCR amplification and result analysis were carried out with reference to Example 1, and the detection of negative and positive quality control products was carried out simultaneously.
[0049] Test result: the amplification curve of the negative quality control product is not S-shaped (se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com