Ethinyloestradiol crude drug impurity, and preparation method and application thereof in serving as standard substance
A technology of butyne and alcohol magnesium bromide, which is applied in the direction of material separation, analysis of materials, steroids, etc., and can solve problems such as unexamined
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
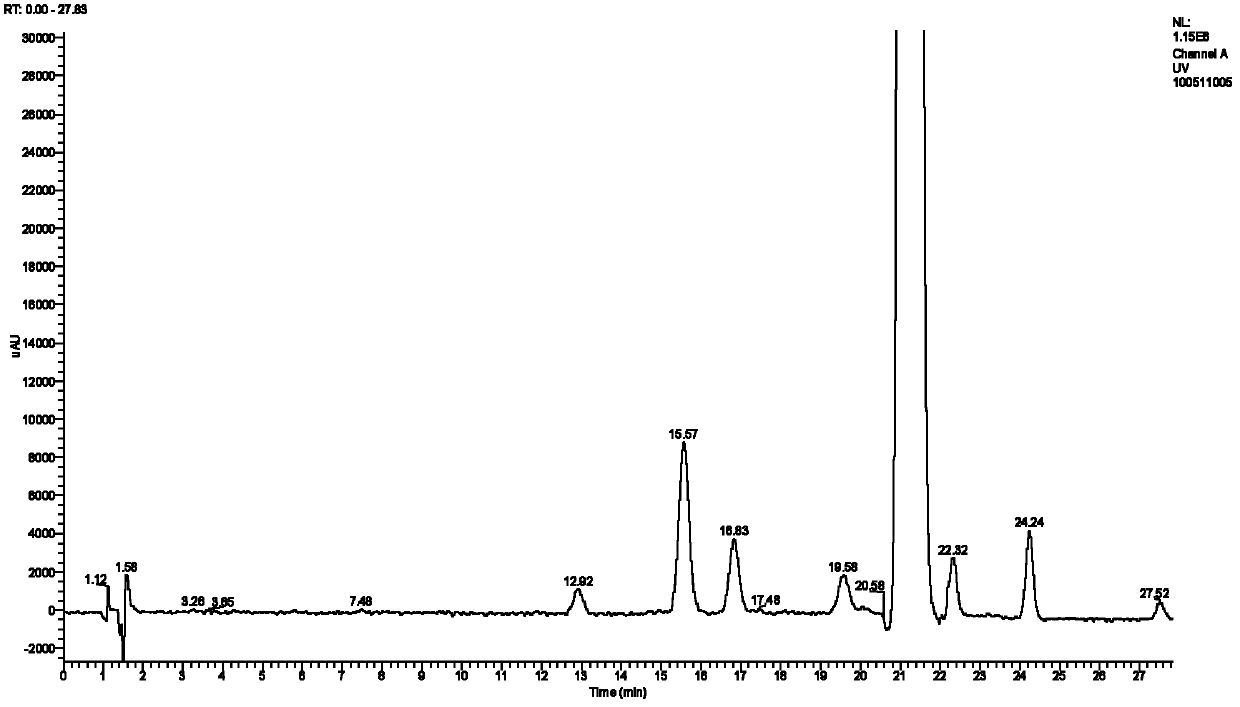
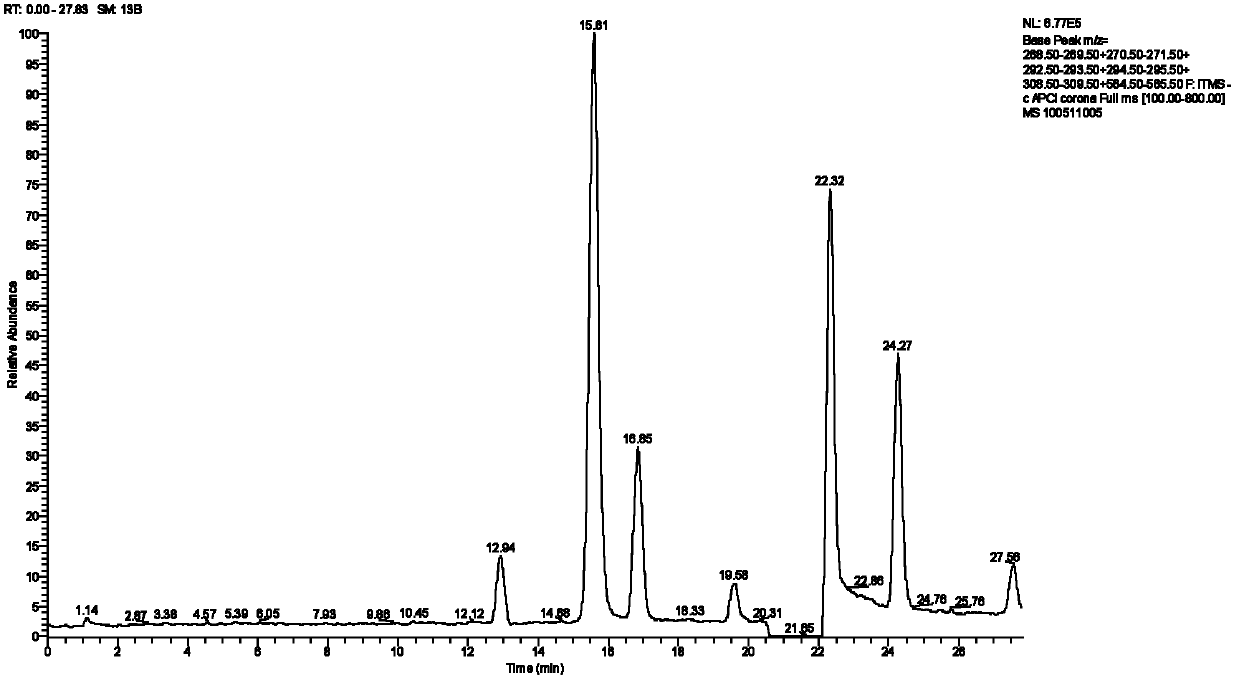
[0024] Chromatographic conditions for the analysis of related substances of ethinyl estradiol:
[0025] Column: Agilent ZORBAX SB C 18 Chromatographic column (150mm×4.6mm i.d., 5μm); mobile phase: water (A) and acetonitrile (B); gradient elution, phase B gradient: (0~20min, 30%~40%, 20~20.5min, 40 %~45%20.5~28min, 45%~50%); flow rate: 1.0mL min -1 ; Column temperature: 30° C.; Injection volume: 10 μL; Detection wavelength: 280 nm.
[0026] Mass Spectrometry Conditions:
[0027] LC-MS analysis: APCI ion source, using negative ion detection mode; nebulizer temperature: 500°C; sheath gas (N 2 ) flow rate: 50arb; auxiliary gas (N 2 ) flow rate: 5arb; exhaust gas sweep (N 2 ) flow rate: 0arb; dissociation current: 4μA; capillary temperature: 150°C; capillary voltage: -12V; tubular lens voltage: -84.28V.
[0028] sample handling
[0029] Accurately weigh 3.0 mg of EE fine drug, dissolve it in 1 mL of methanol, vortex and mix well, and filter through a 0.45 μm microporous memb...
Embodiment 2
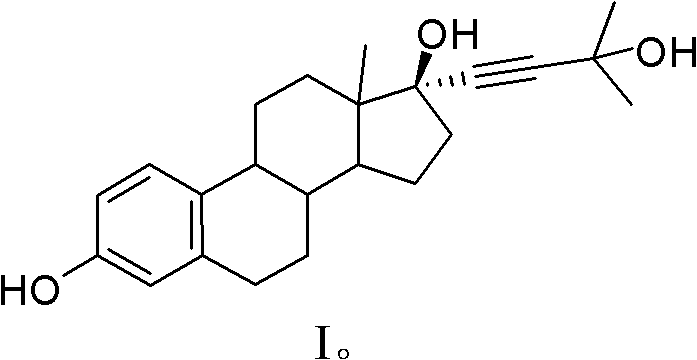
[0037] Magnesium (2640mg, 110mmol) was placed in a dry 500ml three-necked bottle, added to 50ml of anhydrous THF, and N 2 , add a small amount of bromoethane, then add a grain of I 2 , heated to 50°C, until bubbles appeared, and the color of the solution gradually disappeared, continued to add dropwise anhydrous THF diluent of ethyl bromide (7.5ml, 100mmol), kept the solution slightly boiling, and continued the reaction until the magnesium chips were basically After disappearing, add 2-methyl-3-butyn-2-ol (9.6ml, 100mmol) dropwise in anhydrous THF diluent under heating to reflux, and the reaction solution releases gas. After cooling down to room temperature, a solution of estrone (13.5 g, 50 mmol) in anhydrous THF was added dropwise, and the color of the reaction solution gradually turned milky yellow, and the reaction was continued for 3 hours. After the reaction is complete, add NH 4 The aqueous solution of Cl was quenched, THF was distilled off, and ether was added for ex...
Embodiment 3
[0042] The specific method is as example 1, magnesium (2640mg, 110mmol) is put in the dry 500ml three-neck bottle, is added in 50ml anhydrous THF, feeds N 2 , add a small amount of bromoisopropane, then add a grain of I 2 , heated to 50°C, until bubbles appeared, and the color of the solution gradually disappeared. Continue to add the anhydrous THF diluent of bromoisopropane (7.5ml, 100mmol) dropwise, keep the solution slightly boiling, and continue the reaction after the dropwise addition is completed. After the magnesium chips basically disappear, add 2-methyl - Anhydrous THF dilution of 3-butyn-2-ol (9.6ml, 100mmol), the reaction solution emits gas, after reacting for 1 hour, after no longer emitting gas, it is cooled to room temperature, and estrone (13.5g , 50mmol) in anhydrous THF solution, the color of the reaction solution gradually turned milky yellow, and the reaction was carried out for 3h. After the reaction is complete, add NH 4 The aqueous solution of Cl was q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com