Oestrone derivative, immunogen, antibody, enzyme-labeled conjugate, detection reagent and preparation method thereof
A technology of derivatives and conjugates, applied in the fields of immunogens, antibodies, estrone derivatives, enzyme-labeled conjugates, detection reagents and their preparation, which can solve problems such as errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
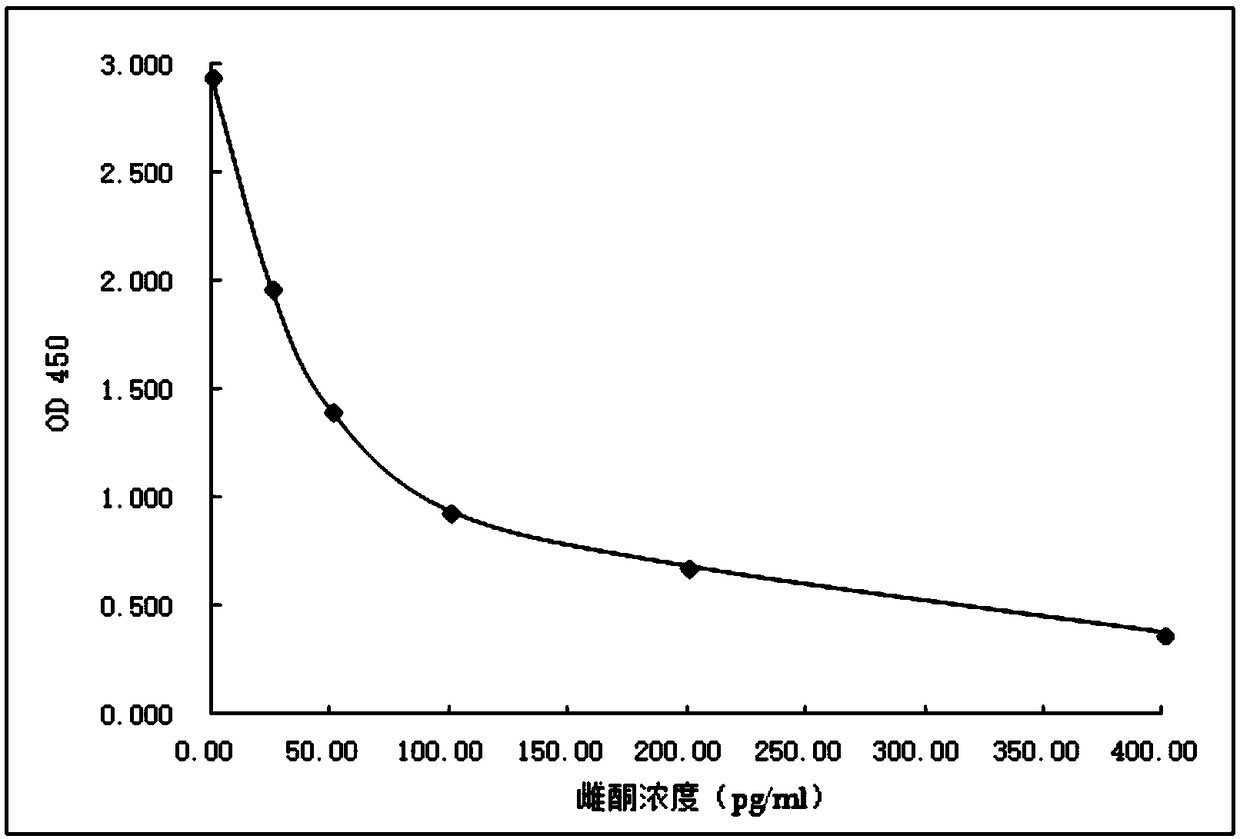
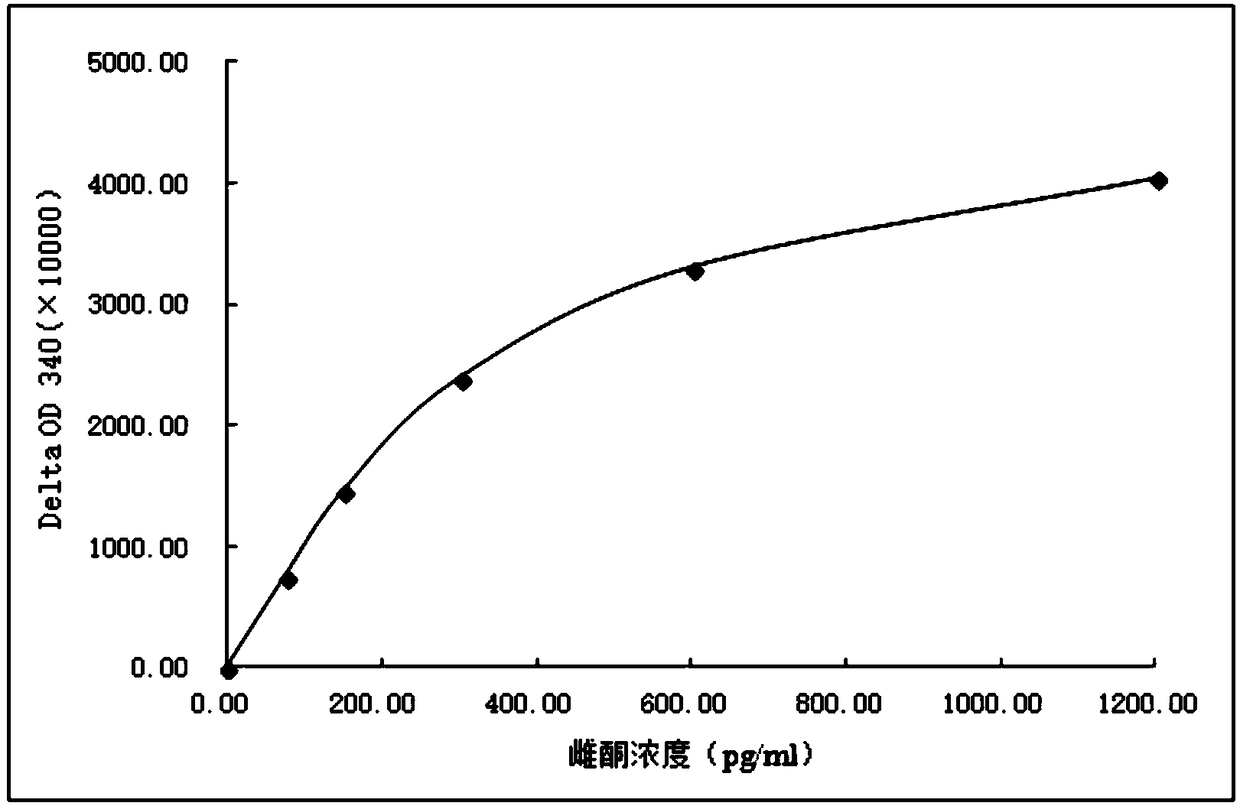
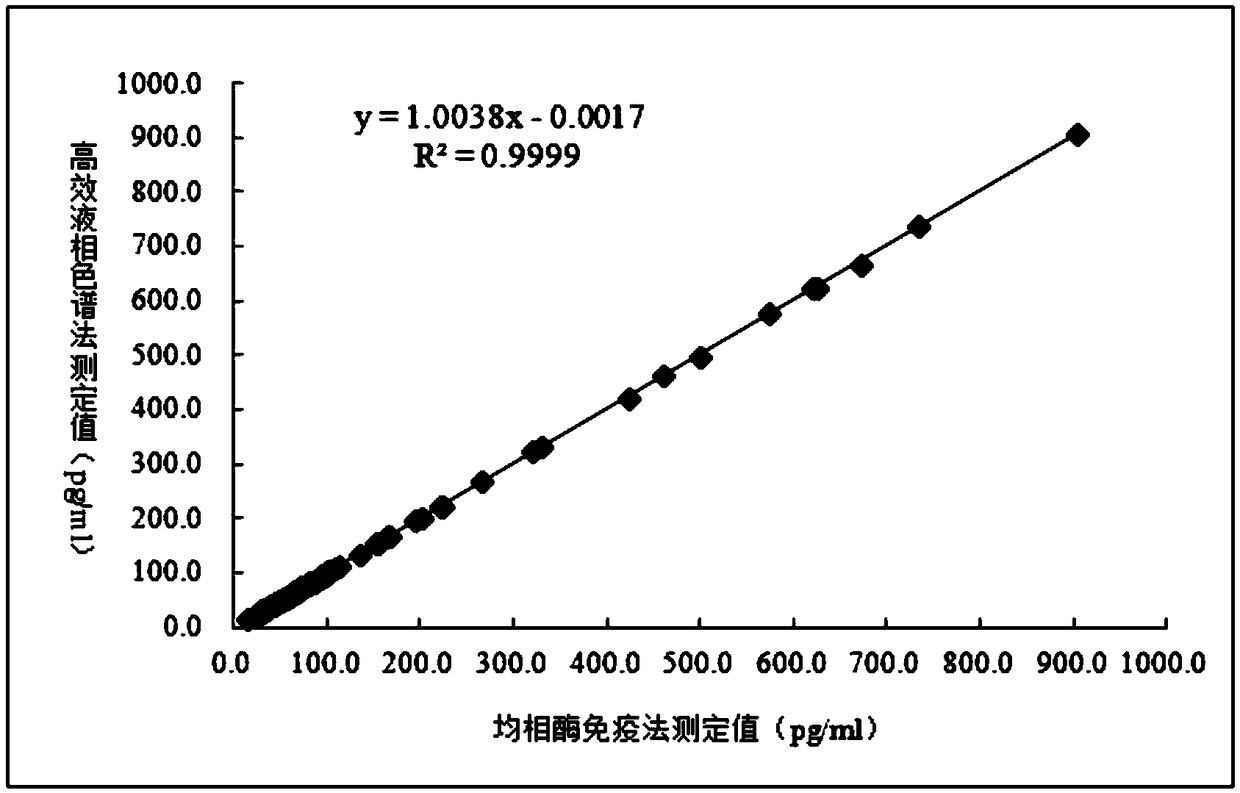
Image
Examples
Embodiment 1
[0080] Example 1. Synthesis of Estrone Derivatives
[0081] The chemical structure of estrone derivatives is shown in formula (I):
[0082]
[0083] The synthetic route and preparation steps of the above-mentioned estrone derivatives are as follows:
[0084]
[0085] The specific synthesis steps are as follows:
[0086] (1) Synthesis of compound 3
[0087]
[0088] Weigh 5.0g (0.018mol) of compound 1 and 10g (0.048mol) of compound 2 and dissolve them in 100mL of acetone, then add 7.9g (0.057mol) of K 2 CO 3 , Make a reaction mixture solution, and then heat the reaction mixture solution to reflux for 12 hours. The solution after the reaction was filtered, and the residue obtained after removing the solvent was purified by a silica gel purification column to obtain 3.0 g of compound 3 as a white solid.
[0089] (2) Synthesis of estrone derivatives
[0090]
[0091] Weigh 3.0g (7.54mmol) of compound 3, 1.1g (11.3mmol) of compound 4, 2.96g (11.3mmol) of triphenylphosphine (PPh 3 ), dissolved...
Embodiment 2
[0093] Example 2. Synthesis of estrone immunogen
[0094] The estrone immunogen in this example is formed by linking the estrone derivative shown in the formula (I) of Example 1 with bovine serum albumin (BSA), and its structural formula is shown in the following formula (II):
[0095]
[0096] The carrier is bovine serum albumin.
[0097] The specific steps of the synthetic method of the estrone immunogen are as follows:
[0098] (1) Weigh 2.72g potassium dihydrogen phosphate, 4.26g disodium hydrogen phosphate, 8.5g sodium chloride, 0.95g magnesium chloride, and dissolve them together in 1L of deionized water, adjust the pH to 8.2 to prepare buffer solution A;
[0099] (2) Weigh 3 mg of BSA and dissolve it in 3 mL of the above buffer solution A at -4°C to make a 1 mg / ml carrier solution;
[0100] (3) Weigh 3 mg of the estrone derivative represented by the above formula (I) and dissolve it in 300 μl of the above buffer solution A at -4°C to prepare a 10 mg / ml estrone derivative solution;...
Embodiment 3
[0103] Example 3 Synthesis of estrone immunogen
[0104] The estrone immunogen in this example is formed by linking the estrone derivative shown in the formula (I) of Example 1 with bovine serum albumin (BSA), and its structural formula is shown in the following formula (II):
[0105]
[0106] The carrier is bovine serum albumin.
[0107] The specific steps of the synthetic method of the estrone immunogen are as follows:
[0108] (1) Weigh 2.3g of potassium dihydrogen phosphate, 3.8g of disodium hydrogen phosphate, 8.0g of sodium chloride, and 0.7g of magnesium chloride, and dissolve them in 0.9L of deionized water, adjust the pH to 8.0, and prepare buffer solution A;
[0109] (2) Weigh 6 mg of BSA and dissolve it in 3 mL of the above buffer solution A at -4°C to make a 2 mg / ml carrier solution;
[0110] (3) Weigh 2.4 mg of the estrone derivative represented by the above formula (I), and dissolve it in 300 μl of the above buffer solution A at -4°C to prepare an 8 mg / ml estrone derivative...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com