Preparation method of propyl o-fluorobenzene compound
The technology of a compound and fluorobenzene is applied in the field of preparation of propyl-o-fluorobenzene compounds, can solve the problems of low product purity, polluted fluorine impurities, high raw material price, etc., and achieves low price, low cost and reduced raw material cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] A kind of preparation method of propyl-o-fluorobenzene compound, first make raw material compound I into lithium reagent, then lithium reagent and allyl chloride or allyl bromide take place coupling reaction under the catalysis of copper, the product obtained is passed through Catalytic hydrogenation can give propyl-o-fluorobenzene compounds.
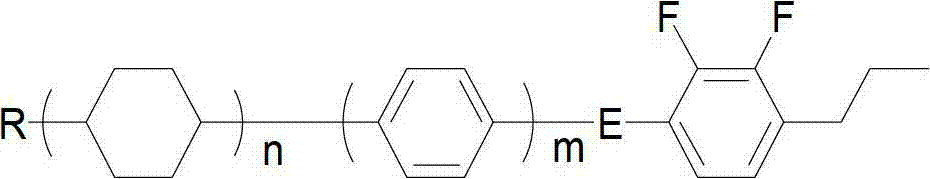
[0060] Wherein, the structural formula of the raw material compound I is:
[0061]
[0062] The R is C 0 ~C 5 straight chain alkyl, said n is 0 or 1 or 2, said m is 0 or 1 or 2, said E is 0 or -CH 2 CH 2 -.
[0063] The specific process is as follows:
[0064]Step 1: Dissolving compound I with an ether solvent, adding alkyllithium dropwise into the solution of compound I at low temperature (-80°C to -20°C) for reaction, while keeping it warm for a period of time (0.5 to 3 hours), Compound II is obtained,
[0065]
[0066] The R is C 0 ~C 5 straight chain alkyl, said n is 0 or 1 or 2, said m is 0 or 1 or 2, said E i...
Embodiment 1
[0077] Preparation of 2,3-difluoro-4-allyl-4′-(trans-4-propylcyclohexyl)-1,1′-biphenyl
[0078] 500ml three-necked bottle, install a thermometer, mechanical stirring, constant pressure dropping funnel, add 31.4g (0.1mol) 2,3-difluoro-4′-(trans-4-propylcyclohexyl)-1,1′- Biphenyl, 188.4g tetrahydrofuran, stirring to cool down to -60°C, dropwise adding 36.5g n-butyllithium, after the dropwise addition, keep warm for 1 hour, add dropwise 11.5g allyl chloride below -50°C, add 0.2g CuCl 2 , -50 ℃ insulation 0.5h, the reaction is complete.
[0079] Add 50g of water dropwise, stir and raise the temperature to 25°C, separate layers, extract the aqueous phase with a small amount of toluene, combine the organic phases, wash with water until neutral, concentrate to obtain 35.4g of crude product, recrystallize 3 times from absolute ethanol to obtain 25.5g of product, yield 72 %, the purity tested by gas chromatography is 99.69%, the melting point mp is 57.6°C, and the clearing point CP is...
Embodiment 2
[0085] Preparation of 2,3-difluoro-4-propyl-4'-(trans-4-pentylcyclohexyl)-1,1'-biphenyl
[0086] According to Example 1, 2,3-difluoro-4'-(trans-4-pentylcyclohexyl)-1,1'-biphenyl and allyl chloride are used as raw materials to obtain 2,3-difluoro- 4-Propyl-4'-(trans-4-pentylcyclohexyl)-1,1'-biphenyl, its purity is 99.9% by gas chromatography, its crystal phase is nearly The crystal phase is 59.4°C, the smectic phase to nematic phase is 88.7°C, and the nematic phase to isotropic phase (clearing point CP) is 123.0°C. The characteristic ion M / Z is tested by mass spectrometry (GC-MS) + for
[0087] 384,355,328,313,273,258,229,203,183,166,151,127,109,81,55.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com