Isothermal nucleic acid amplification reaction reagent and isothermal nucleic acid amplification method
An isothermal nucleic acid amplification and reaction reagent technology, applied in the field of high-sensitivity nucleic acid amplification technology, can solve the problems of large power consumption, cost and application range limitations, and achieve the effect of simple operation, simple operation and low technical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Detection of foot-and-mouth disease virus (FMDV) antigen VP1 gene transgenic tobacco
[0036] First, the FMDV antigen VP1 gene is inserted into the vector p16APT containing the tobacco chloroplast-specific Prrn promoter and the psbA terminator to form the expression cassette of the target gene; then the complete expression cassette is excised by restriction enzymes and Inserted into the vector pTRV containing homologous fragments rpl2-trnH-psbA and trnK-ORF509A of tobacco chloroplast genome to form a complete plasmid expressed by tobacco chloroplast containing FMDV antigen VPI gene.
[0037]On the other hand, cultivate aseptic tobacco (Nicotiana tabacum), take aseptic tobacco seedlings at the 4-6 leaf stage, select expanded leaves of aseptic tobacco seedlings with a diameter of about 3 cm as explants, and place them on a 9 cm sterile culture dish center. There is a thin layer of MS medium containing 0.2mol / L mannitol and 0.2mol / L sorbitol in the petri dish, w...
Embodiment 2
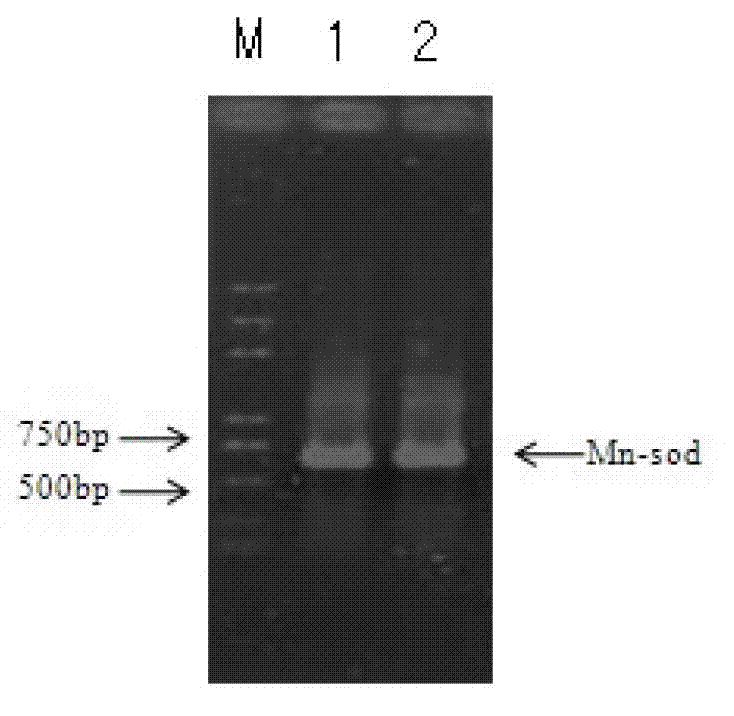
[0106] Example 2 Detection of Mn-SOD Gene Transgenic Tobacco
[0107] In this example, the superoxide dismutase (Mn-SOD) gene was first inserted into the vector pRTL2 containing the tobacco cell nucleus E35S promoter, TEV Leader and 35S terminator to form the expression cassette of the target gene. Then the complete expression cassette is inserted into the tobacco transformation vector Pzp211 through the action of the restriction endonuclease pstI to form a complete plasmid expressing in the tobacco cell nucleus containing the superoxide dismutase (Mn-SOD) gene.
[0108] On the other hand, cultivating aseptic tobacco seedlings: sow the seeds of tobacco leaves on MS medium under aseptic conditions, and take the young leaves of the aseptic seedlings for transformation after about 6-8 weeks. Tobacco leaf discs were infected with Agrobacterium containing the recombinant plasmid. Sterile tobacco leaf discs need to be pre-cultured on MS1 solid medium for 3 days. After the Agroba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com