For the treatment of gastrointestinal disorders
A technology for gastrointestinal diseases and diseases, which is applied in the field of peptides and compositions for the treatment of upper gastrointestinal diseases, and can solve problems such as limited efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
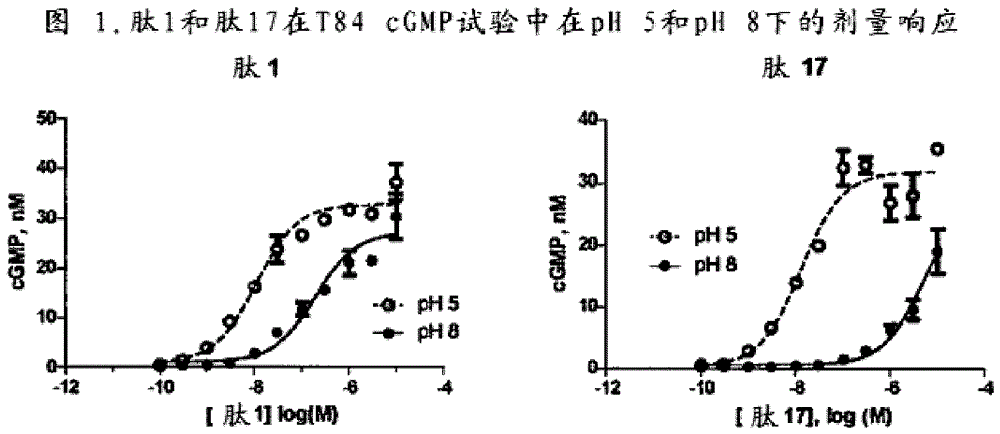
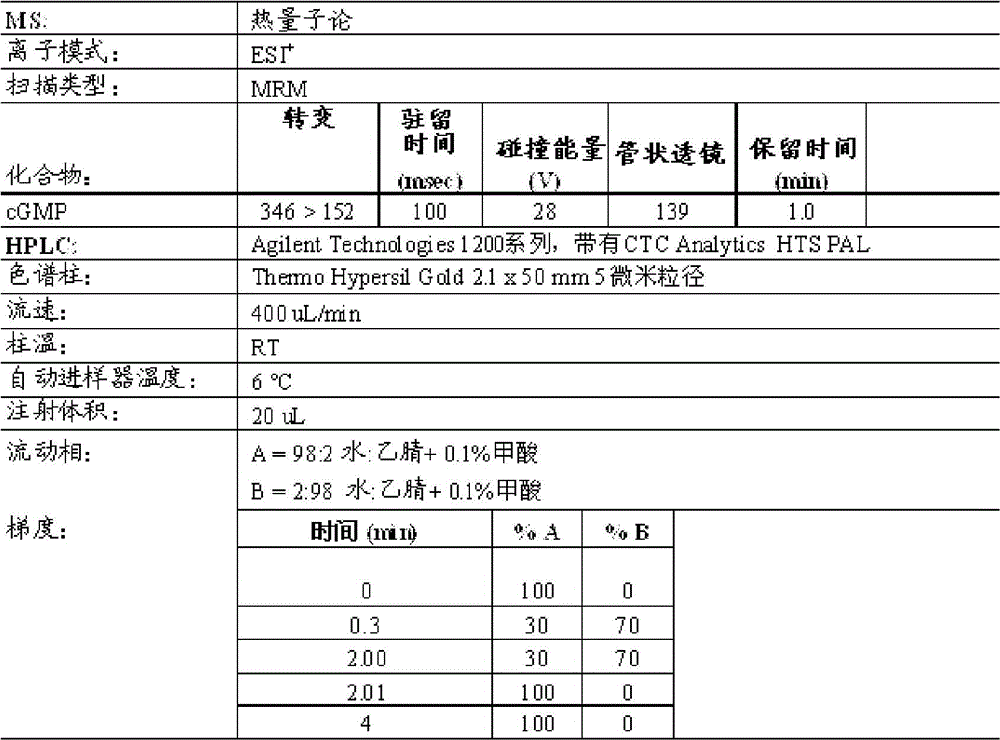
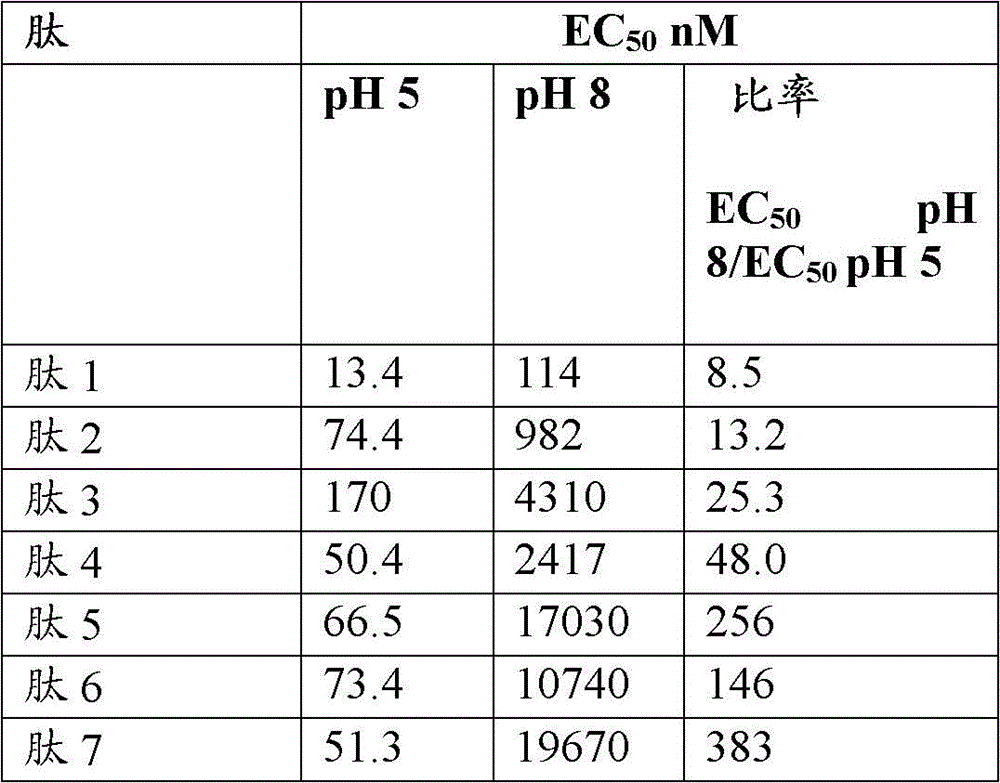
[0402] Example 1: Accumulation of cGMP in T84 cells for GC-C activity analysis
[0403] GC-C activity of cGMP accumulation in T84 cells was analyzed. For cGMP testing, make 4.5 x 10 5 cells / mL of T84 cells were grown overnight in 24-well tissue culture plates. The next day, T84 cells were washed twice with 1 mL of DMEM + 20 mM MES (pH 5) or DMEM + 50 mM sodium bicarbonate (NaBicarb, pH 8), where these buffers do not contain serum. After the second wash, cells were incubated with 450 μL of 1 mM isobutylmethylxanthine (IBMX) in pH 5 or pH 8 buffer for 10 minutes at 37° C. to inhibit any phosphodiesterase activity. Peptides were then diluted to 10x concentration in pH 5 or pH 8 buffer. Dilute 50 µL of the peptide solution with T84 cells to a final volume of 500 µL so that the individual peptide concentrations are 1x. Eleven point curve analysis (in nM) was performed for each peptide tested in each assay at final peptide concentrations: 10000, 3000, 1000, 300, 100, 30, 10, 3...
example 2
[0416] Example 2: Competitive radioligand binding on T84 cells
[0417] Competitive radioligand binding assays were performed using intact human T84 cells from the American Type Culture Collection (ATCC; Manassas, VA). Make T84 cells in Dulbecco's Modified Eagle Medium (Dulbecco's Modified Eagle Medium): Ham's F-12 50 / 50 medium (DMEM / F12)+5% fetal bovine serum (FBS) in T Grow as a monolayer on -150 plastic bottles to 60-70% confluence. Cells were harvested by lightly scraping with a cell scraper and collected by centrifugation at 2000g for 10 minutes at 4°C. Cells were washed twice by gently resuspending in phosphate buffered saline (PBS) and harvested by centrifugation as described above.
[0418] By dissolving 100 μg of NTFYCCELCCCNPACAGCY (SEQID NO: 71) (Enterotoxin STp; Bachem H-6248) in 0.5 mL of water, it was sent to Perkin-Elmer Life and Analytical Sciences (N. Billerica, MA) for use in Marchanolis, J.J., "An enzymic method for the traceiodination of immunoglobulin...
Embodiment 3
[0428] Example 3: Gastrointestinal transit in mice
[0429] The purpose of this assay is to examine the effect of guanylate cyclase C peptide on gastrointestinal transit in vivo in mice. Orally administered doses of guanylate cyclase C agonists have been shown to increase the % stroke of a charcoal meal in mice. The assay may be a marker for the activity of the described guanylate cyclase C peptide to enhance upper GI transport in the patient's small intestine.
[0430] For the experiments, female CD-1 mice (n=10 per group) weighing 25-30 g were fasted overnight and given ad libitum water. Activated charcoal (20 g; 100 mesh; Sigmacat #242276) was suspended in 200 mL of gum arabic (100 mg / mL) and stirred for at least one hour. Detection peptides were prepared in 20 mM Tris (pH 6.9) coal medium.
[0431] A dose of 200 [mu]L of detection peptide and kerosene was administered by oral gavage. Seven minutes after administration of the test peptide, 200 μL of the charcoal / gum a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com