Ferric fluoride nano material and preparation method thereof
A nanomaterial, iron trifluoride technology, applied in the direction of iron halide, nanotechnology, electrical components, etc., can solve the problems of high consumables, large FeF3 particle size, etc., to achieve high yield, high practical application value, high crystallinity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0020] The preparation method of embodiment 1 iron trifluoride nanometer material
[0021] Include the following steps:
[0022] (1) 0.1263g Fe(NO 3 ) 3 9H 2 O was dissolved in 30mL of absolute ethanol to obtain a clear alcoholic solution containing iron, in which the concentration of iron ions was 10.4mM;
[0023] (2) Under stirring conditions, 0.0267g NH 4 HF 2 Add in the above-mentioned iron-containing alcohol solution until the system becomes a colorless and transparent solution, and the solution is transferred to a polytetrafluoroethylene reactor with a volume of 50mL; the Fe(NO 3 ) 3 9H 2 O and NH 4 HF 2 The molar ratio is 1:1.5 (the molar ratio of iron ion to fluoride ion is 1:3);
[0024] (3) Heat-treat the hydrothermal reactor at 80°C for 4 hours, cool naturally, centrifuge, wash with ethanol three times, and vacuum dry at 60°C for 10 hours to obtain FeF 3 0.33H 2 O nanomaterials.
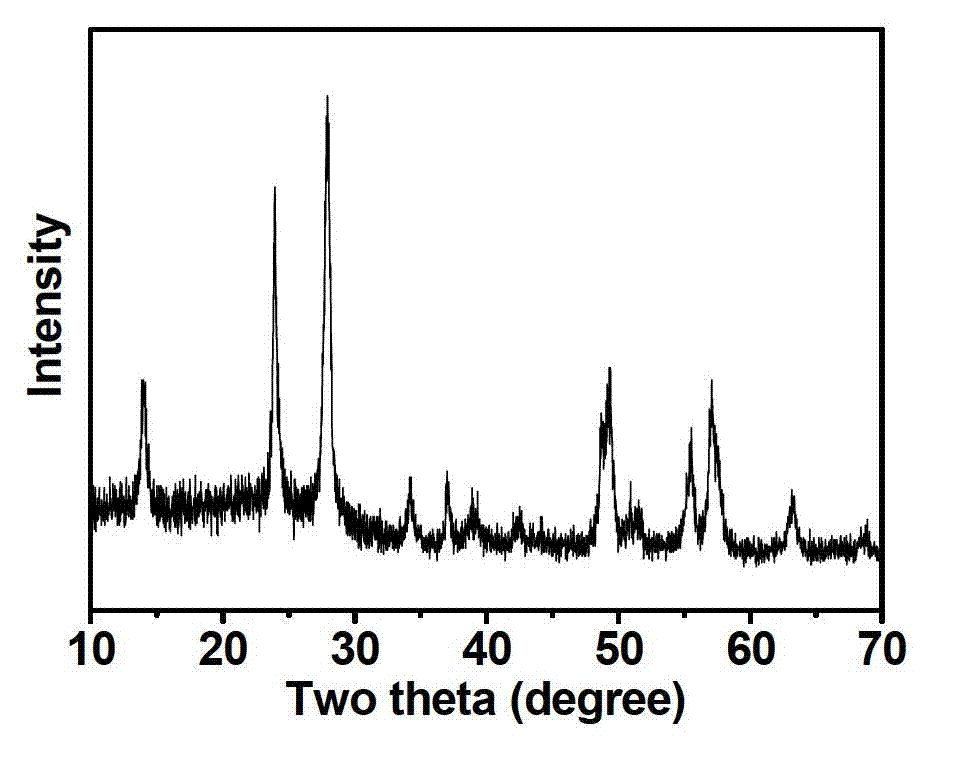
[0025] To the FeF that embodiment 1 prepares gained 3 0.33H 2 O nanomater...
Embodiment 2 3
[0027] The preparation method of embodiment 2 iron trifluoride nanometer material
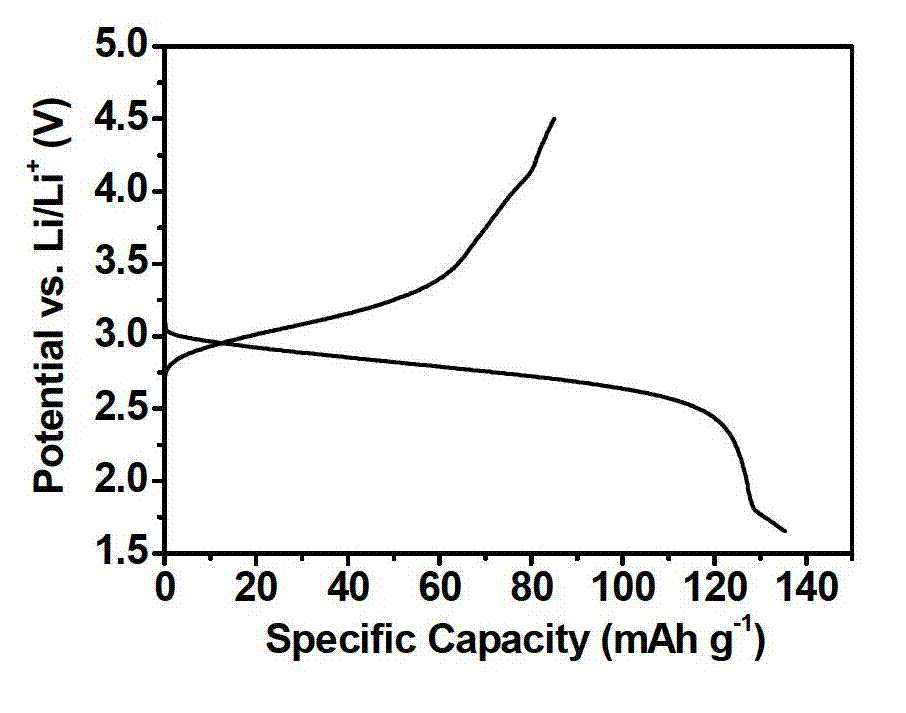
[0028] Preparation method is the same as embodiment 1, just Fe(NO 3 ) 3 9H 2 The consumption of O becomes 0.606g (in the alcohol solution containing iron, the concentration of iron ion is 50mM), the NH 4 HF 2 The dosage becomes 0.1485g(Fe(NO 3 ) 3 9H 2 O and NH 4 HF 2 The molar ratio of the iron ion to the fluorine ion is 1:1.5, and the molar ratio of the iron ion to the fluoride ion is 1:3), and the XRD spectrum shows that FeF 3 0.33H 2 O nanomaterials. The particle size is about 10-15 nanometers, and the specific discharge capacity of the material is 130mAh / g.
Embodiment 3 3
[0029] The preparation method of embodiment 3 iron trifluoride nanometer material
[0030] Preparation method is the same as embodiment 1, just Fe(NO 3 ) 3 9H 2 The consumption of O becomes 1.212g (in the alcohol solution containing iron, the concentration of iron ion is 0.1M), the NH 4 HF 2 The dosage becomes 0.297g(Fe(NO 3 ) 3 9H 2 O and NH 4 HF 2 The molar ratio of the iron ion to the fluorine ion is 1:1.5, and the molar ratio of the iron ion to the fluoride ion is 1:3), and the XRD spectrum shows that FeF 3 0.33H 2 O nanomaterials. The particle size is about 10-15 nanometers, and the discharge specific capacity of the material is 122mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com