Chloroquinoxaline compound
A kind of fluoroquinoxaline, substituted quinoxaline technology, applied in the field of fluoroquinoxaline compounds, can solve the problems such as no reports on preparation method and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Compound A
[0048] Follow the reaction equation shown in Scheme 2.
[0049]
[0050] Reaction equation of Scheme 2 compound A
[0051] (1) Preparation of 4-fluoro-3,6-dibromo-1,2-phenylenediamine (compound 1)
[0052] 5-Fluoro-4,7-dibromo-2,1,3-benzothiadiazole (5 g, 0.016 mol) was dissolved in 150 ml of absolute ethanol, and NaBH was added in batches at 0 °C 4 (11.1g, 0.29mol), and then reacted at room temperature for 20h. After the reaction, concentrate to remove ethanol, add 160ml of water, extract with ethyl acetate, wash the organic phase with saturated brine, and finally anhydrous MgSO 4 dry. The crude product obtained after concentrating and removing the organic solvent was subjected to silica gel column chromatography with n-hexane / ethyl acetate (25:1) as the eluent to obtain 4-fluoro-3,6-dibromo-1,2-phenylenediamine 3.5 g (compound 1), yield 78%.
[0053] (2) Preparation of 1,2-bis(3-octyloxyphenyl)ethanedione (compound 2)
[0054] Add LiB...
Embodiment 2
[0059] Example 2: Compound B
[0060] Follow the reaction equation as shown in Scheme 3.
[0061]
[0062] Reaction equation of Scheme 3 compound B
[0063] (1) Synthesis of 6-fluoro-5,8-dithiophene-2,3-bis(3-octyloxyphenyl)quinoxaline (compound 3)
[0064] Compound A (0.821g, 1.15mmol), 2-tributyltinthiophene (0.918g, 2.46mmol) and Pd(PPh 3 ) Cl 2 (32mg, 0.046mmol) dissolved in 20ml toluene, N 2 Reflux reaction under protection for 12 hours. The toluene was concentrated, and the crude product was recrystallized from n-hexane to obtain 0.66 g of 6-fluoro-5,8-dithiophene-2,3-bis(3-octyloxyphenyl)quinoxaline (compound 3) as an orange solid. The rate is 80%.
[0065] (2) Synthesis of 6-fluoro-5,8-bis(5-bromothiophene)-2,3-bis(3-octyloxyphenyl)quinoxaline (compound B)
[0066] Compound 3 (0.5g, 0.7mmol) and NBS (0.261g, 1.47mmol) were dissolved in DMF (20ml), heated to 40°C, kept for 7h, cooled, filtered, and the filter cake was washed with methanol to obtain 6-fluoro -...
Embodiment 3
[0069] Example 3: Compound C
[0070] Follow the reaction equation as shown in Scheme 4.
[0071]
[0072] Reaction equation of Scheme 4 compound Ⅳ
[0073] Using compound A and 4-hexyl-2-tributyltinthiophene as raw materials, compound C was prepared according to the method for synthesizing compound B with a yield of 65%.
[0074] NMR characterization data: 1 HNMR (500MHz, CDCl 3 , ppm): δ=7.91 (d, 1H), 7.72 (s, 1H), 7.55 (dd, 2H), 7.50 (S, 1H), 7.20 (td, 2H), 7.09 (M, 2H), 6.97 ( dt, 2H), 4.05 (q, 4H), 2.64 (td, 4H), 1.80 (m, 4H), 1.66 (m, 4H), 1.49 (dd, 4H), 1.4-1.25 (m, 28H), 0.9 ( m,12H).
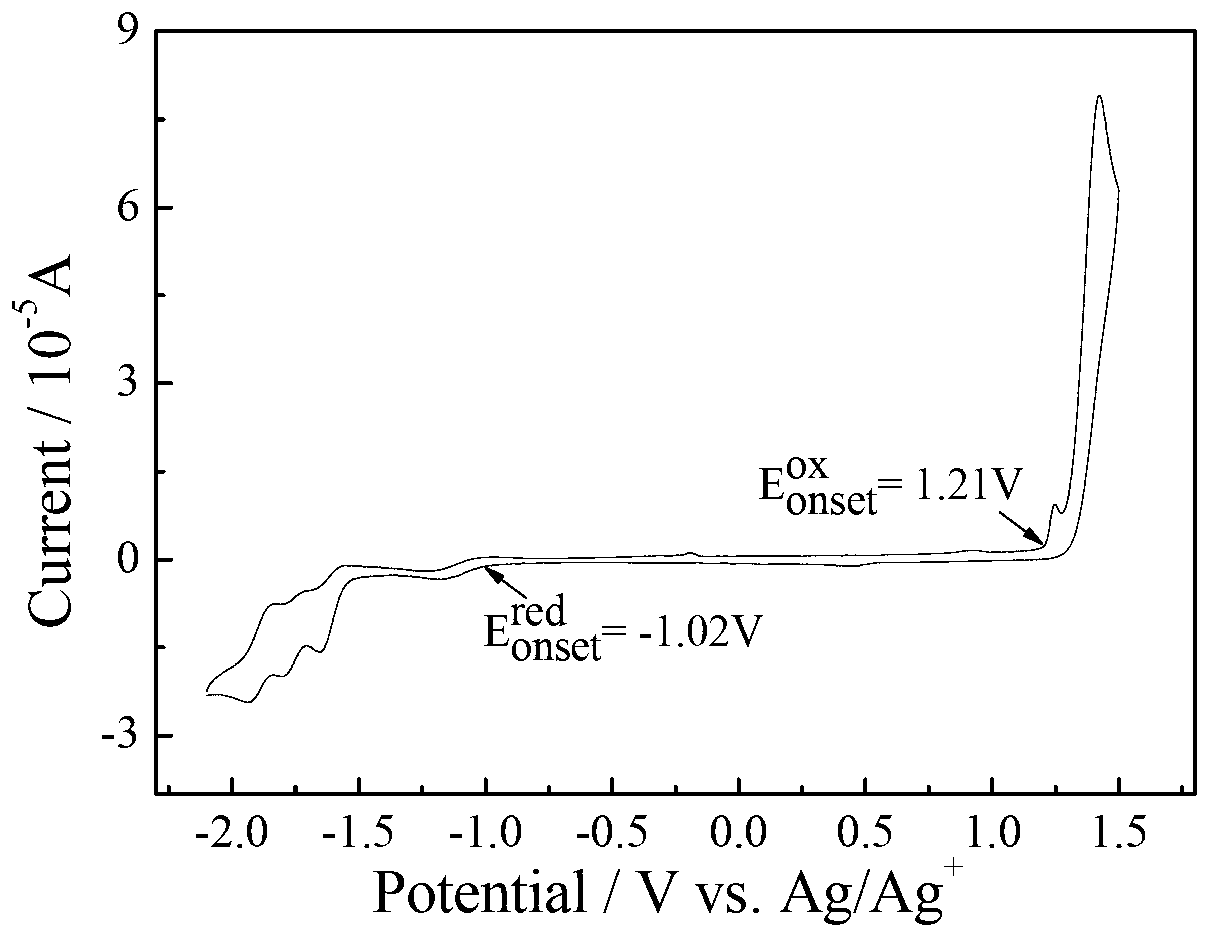
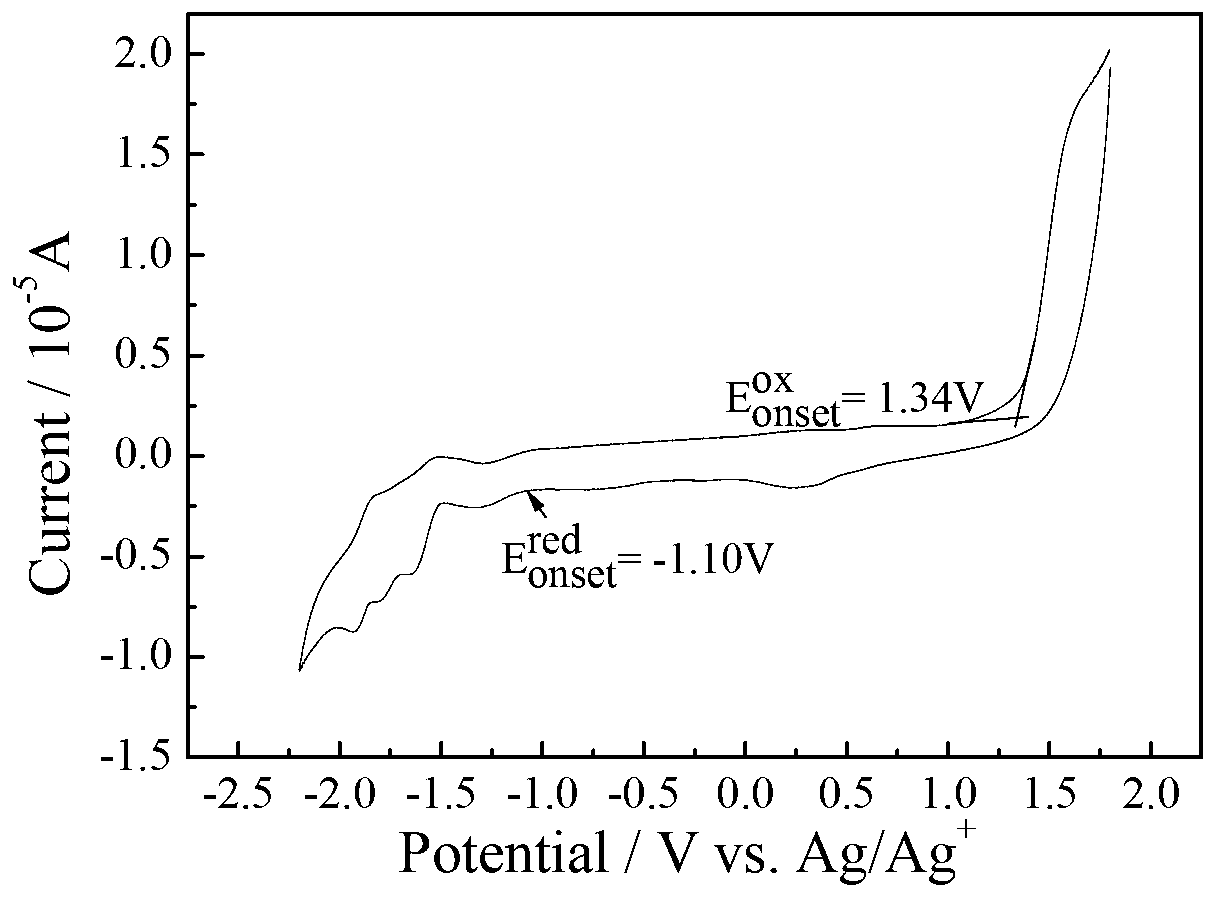
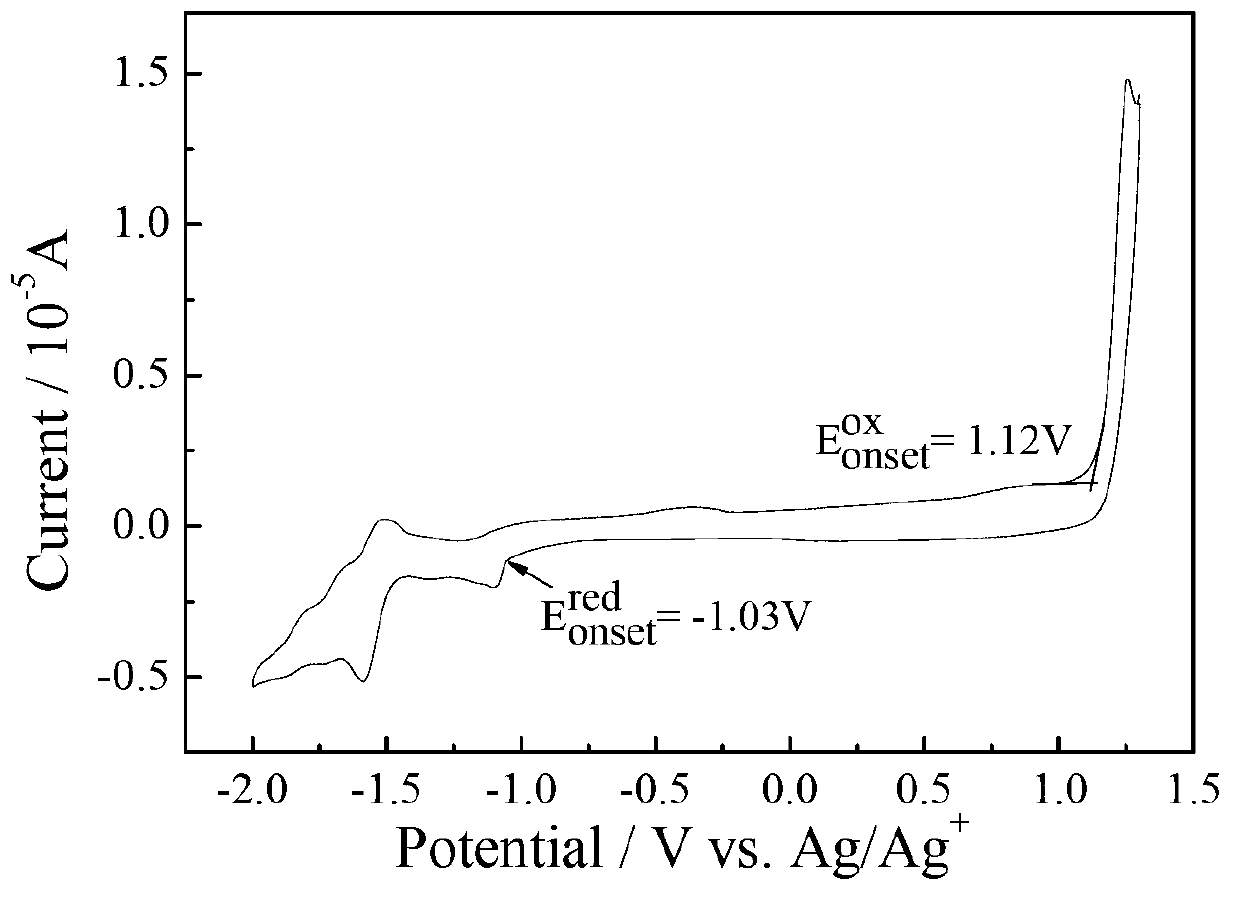
[0075] Electrochemical test: use CHI660D electrochemical workstation, use glassy carbon electrode as working electrode, platinum wire electrode as counter electrode, AgAg + The electrode is the reference electrode, Bu 4 N·PF 6 As an electrolyte, in a solvent of acetonitrile / o-dichlorobenzene=1:5, the HOMO energy of compound C determined by cyclic voltammetry is -5.58eV, which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com