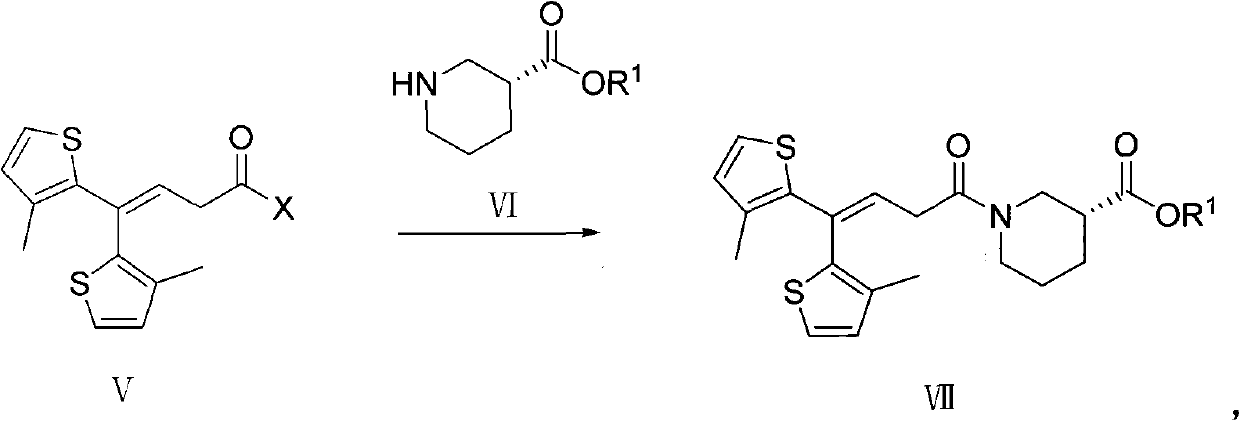
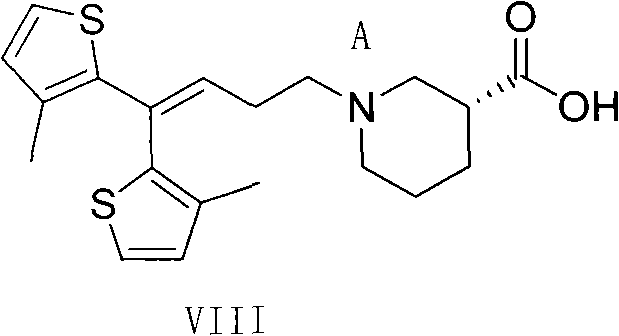
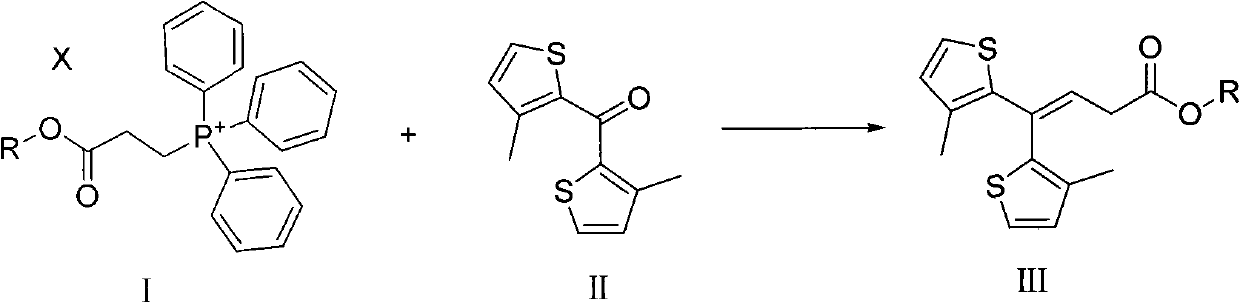
Method for preparing tiagabine and precursor compound of tiagabine
A compound and product technology, applied in the field of drug preparation, can solve the problems of piperidine carboxylate waste, difficulty in large-scale production and low yield of N-alkylation, etc., to reduce production costs, easy product purification, simplify The effect of the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of compounds of formula III (R=C 2 H 5 )
[0056] Under the protection of N2, the compound of formula I (wherein R=C 2 H 5 ) (100.86g, 245mmol) was dissolved in 500ml of dry toluene, the compound of chemical formula II (50.0g, 223mmol) was suspended in 1500ml of dry toluene, and the toluene suspension of the compound of chemical formula II was dropped into chemical formula I under stirring toluene solution of the compound. The mixture was heated to reflux and stirred for 23h. The solution was spin-dried under reduced pressure to give a yellow oil. 1000 ml of n-hexane:acetone (9:1) was added to the oil, stirred, filtered, and the filtrate was concentrated to obtain 70.67 g of the compound of formula III with HPLC purity >80%. The obtained compound of formula III contains partially unremoved triphenylphosphine oxide, which is removed in the next step, and the sample for structure determination is purified by column chromatography (9:1 n-hexane...
Embodiment 2
[0057] Example 2: Preparation of compounds of formula IV
[0058] 12.0 mol / L NaOH (25 mL, 300 mmol) was added to the ethanol (300 mL) solution of the compound of chemical formula III (40.63 g, 151 mmol), stirred at room temperature for 4 h, after TLC monitoring was completed, the reaction was filtered, and the obtained solid was added to 200 ml of water, and the mixture was concentrated. The hydrochloric acid was acidified to pH 4, and a white solid was precipitated, which was stirred at room temperature for 2 h and filtered with suction. The filter cake was washed with water and dried in vacuo to obtain 30.78 g of the compound of formula IV with a yield of 90% and a HPLC purity of 96%. MS(ESI): 277.2; 1 H NMR (500MHz, Chloroform) δ: 2.17 (s, 6H), 3.49 (d, J=7.0 Hz, 2H), 6.53 (s, 1H), 7.19-7.03 (m, 2H), 7.64-7.49 (m , 2H).
Embodiment 3
[0059] Example 3: Preparation of compounds of formula V:
[0060] The compound of chemical formula IV (47.33 g, 170 mmol) was put into a reaction flask, 3000 ml of freshly distilled toluene was added, and the mixture was stirred to dissolve. After cooling in an ice bath, 20 ml of N,N-dimethylformamide was added dropwise. Keeping the temperature of the reaction system, 560 ml of thionyl chloride was slowly added dropwise at 20°C. After the dropwise addition was completed, the reaction was stirred at room temperature for 1 h, and then kept at 50°C for the reaction. The reaction progress was tracked by TLC (conditions: petroleum ether: ethyl acetate = 3:1), the reaction was complete after about 3h. Concentrate under reduced pressure to remove the solvent, add 500 ml of freshly distilled toluene and continue to concentrate until no solvent is concentrated, and the residue is dissolved in 500 ml of THF for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com