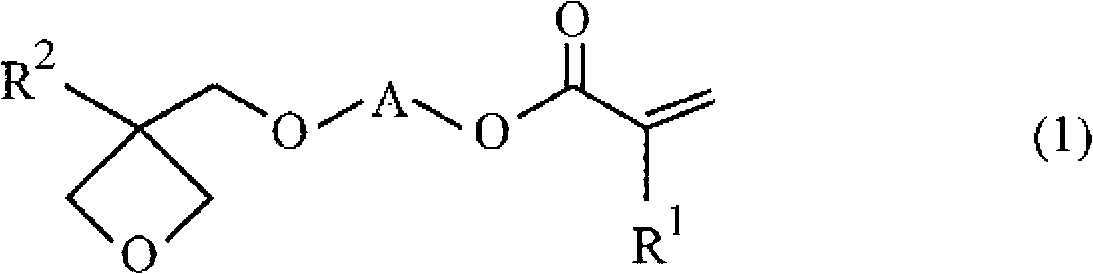
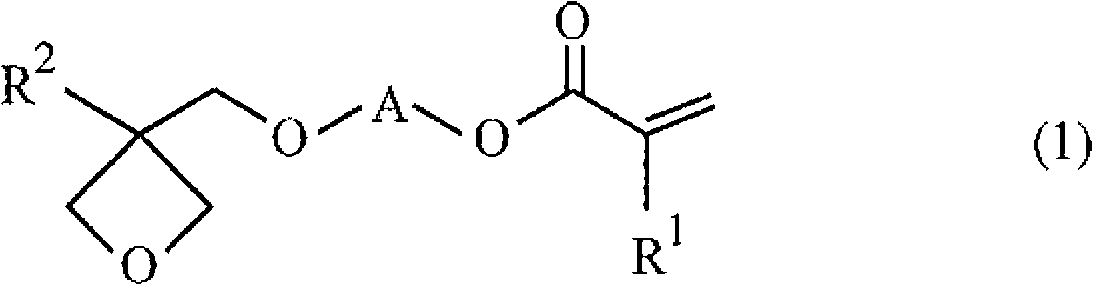
Radical polymerizable resin, radical polymerizable resin composition, and cured product thereof
A technology of resin composition and polymeric compound, which is applied in the field of radically polymerizable resin composition and its cured product, can solve the problems of low curability, insufficient flexibility, toxicity, etc., and achieve thermal deterioration prevention, excellent flexibility, Excellent workability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 (manufacture of radically polymerizable resin)
[0098] Add 3.78 g of toluene and 3-ethyl-3-(3-acryloyloxy-2,2-dimethylpropane represented by the following formula A mixed solution (monomer mixed solution) of 8.82 g (34.67 mmol) of oxymethyl)oxetane (EOXTM-NPAL) and 0.039 g of 4-methoxyphenol was adjusted to 25°C. Next, a liquid mixture of 0.093 g of toluene and 0.016 g (0.11 mmol) of boron trifluoride diethyl ether complex was quantitatively added dropwise over 2 hours using a liquid delivery pump. After the dropwise addition, keep it for 4 hours, then use 5 times the amount of methanol (containing 0.1% 4-methoxyphenol) to carry out precipitation purification to the mixed solution, and keep it in a vacuum dryer (40°C, full vacuum). After 20 hours, a colorless and transparent liquid resin (1) was thus obtained.
[0099] The polystyrene conversion weight average molecular weight of this liquid resin (1) was 24800.
[0100] [chemical formula 7]
[0101]
Embodiment 2
[0102] Example 2 (manufacture of radically polymerizable resin)
[0103] Add toluene 19.98g, EOXTM-NPAL 8.82g (34.67mmol), 3-ethyl-3-(2-ethylhexyloxymethyl ) oxetane (trade name "OXT-212", manufactured by Toagosei Co., Ltd.) 23.56g (0.10mol), 4-methoxyphenol 0.039g mixed solution (monomer mixed solution), and the temperature Adjust to 25°C. Next, a liquid mixture of 5.60 g of toluene and 0.95 g (6.60 mmol) of boron trifluoride diethyl ether complex was quantitatively added dropwise over 2 hours using a liquid delivery pump. After the dropwise addition, keep it for 4 hours, then use 5 times the amount of methanol (containing 0.1% 4-methoxyphenol) to carry out precipitation purification to the mixed solution, and keep it in a vacuum dryer (40°C, full vacuum). After 20 hours, a colorless and transparent liquid resin (2) was thus obtained.
[0104] The polystyrene conversion weight average molecular weight of this liquid resin (2) was 8200.
Embodiment 3
[0105] Example 3 (manufacture of radically polymerizable resin)
[0106] Add toluene 20.40g, EOXTM-NPAL 8.82g (34.67mmol), OXT-212 39.26g (0.17mol), 4-methoxyphenol 0.039g in the three-necked flask that initiator dripping line, nitrogen line, thermometer are installed g of the mixed solution (monomer mixed solution), and adjust the temperature to 25 ° C. Next, a liquid mixture of 5.60 g of toluene and 0.95 g (6.60 mmol) of boron trifluoride diethyl ether complex was quantitatively added dropwise over 2 hours using a liquid delivery pump. After the dropwise addition, keep it for 4 hours, then use 5 times the amount of methanol (containing 0.1% 4-methoxyphenol) to carry out precipitation purification to the mixed solution, and keep it in a vacuum dryer (40°C, full vacuum). After 20 hours, a colorless and transparent liquid resin (3) was thus obtained.
[0107] The polystyrene conversion weight average molecular weight of this liquid resin (3) was 8300.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
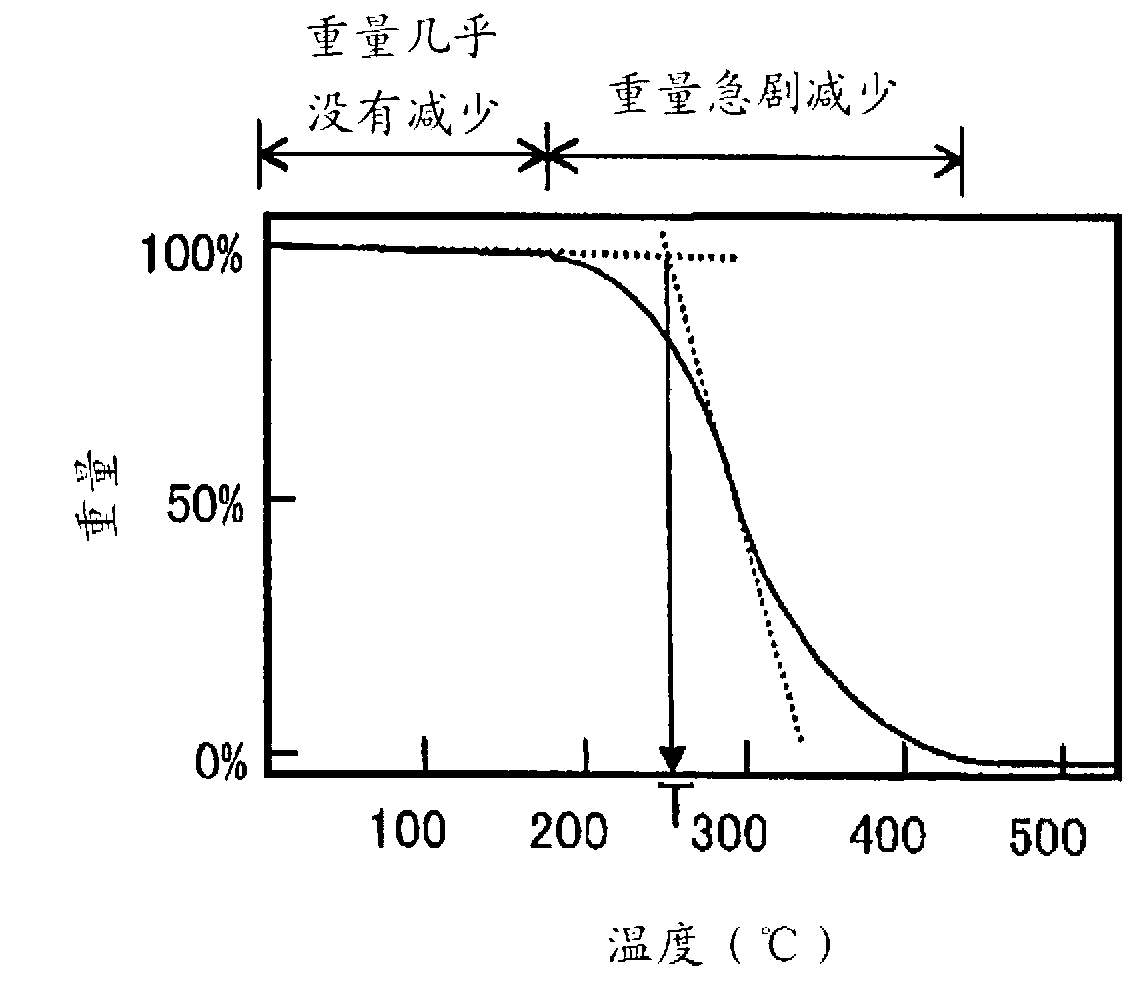
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com