Vaccine for preventing and treating scars and preparation method thereof
A vaccine and scar technology, applied in the field of vaccines, can solve the problems of lack of pathological site specificity, complicated purification process, and many times of medication, so as to prevent and treat scar formation, reduce toxic side effects, and reduce dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Obtaining hIL-10-CBD recombinant gene
[0027] (1) Obtaining hIL-10cDNA
[0028] 1. Extraction of total RNA
[0029] Mononuclear cells were separated from human peripheral blood by lymphocyte separation medium, washed twice with PBS, resuspended in 10% RPMI 1640 culture medium, added ConA with a final concentration of 10g / l, placed in a 5% CO2 incubator, and incubated at 37°C for 48h. Collect cells. According to the method of RNA extraction kit, total RNA was extracted and stored at -80°C for later use.
[0030] 2. Extraction of total RNA and amplification of hIL-10cDNA
[0031] Use the RNA extraction and inversion kit from Takara Company to extract total RNA according to the instructions, and perform RT-PCR reaction: total RNA 500ng, 5× reverse transcription Buffer 2μl, oligo dT 0.5μl, 6mer 0.5μl, AMV reverse transcriptase 0.5μl, DEPC water was added to 10 μl. Incubate at 37°C for 30 minutes, then incubate at 85°C for 3 minutes to obtain cDNA.
[0032]...
Embodiment 2
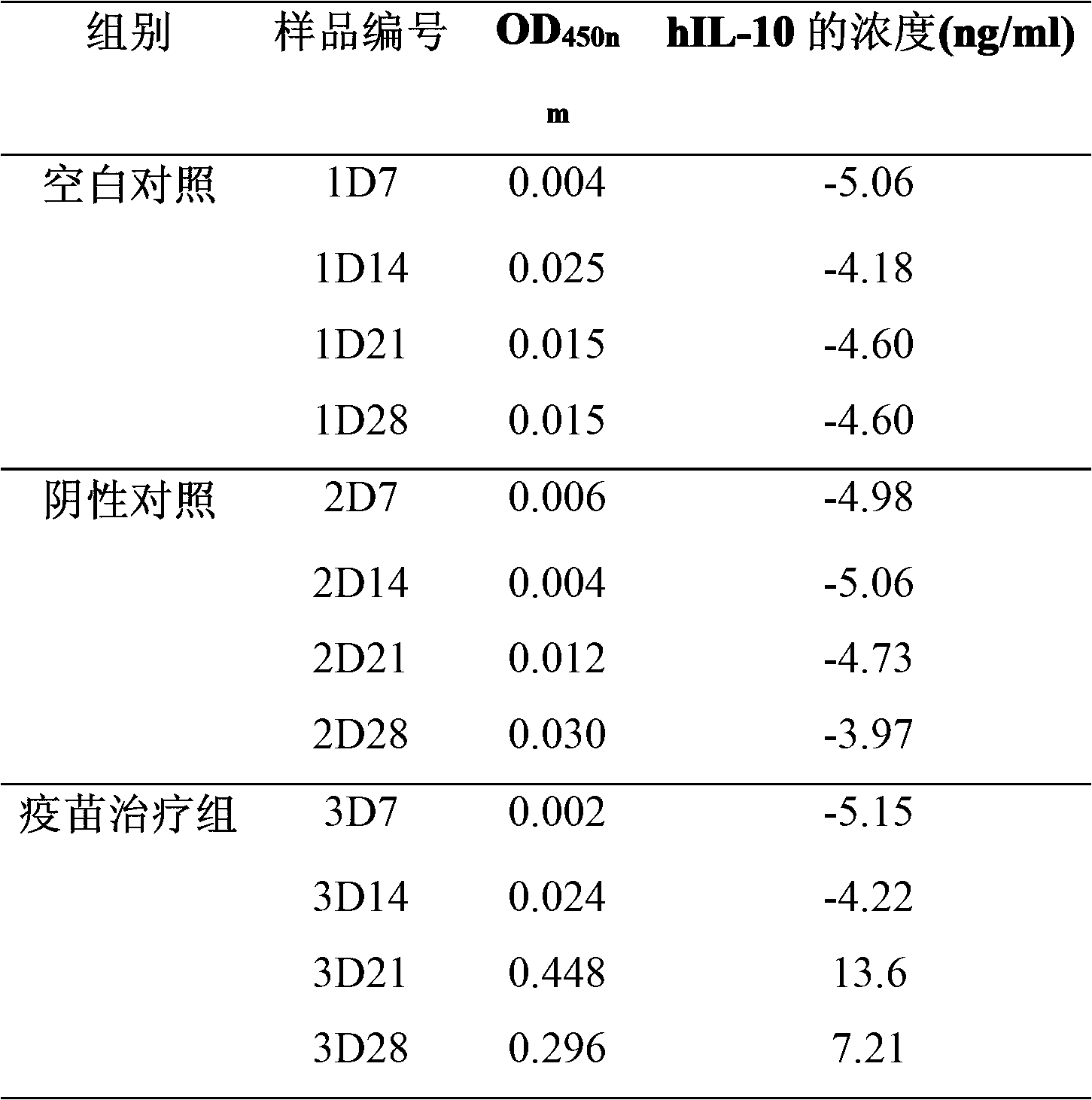
[0043] Embodiment 2: Preparation and quality standard control of nucleic acid vaccine
[0044] (1) Preparation of vaccine
[0045] 1. Vaccine Amplification
[0046] The above-mentioned pcDNA-hIL10-CBD / DH5α bacteria were inoculated in LB medium containing 100 μg / ml ampicillin, cultured with shaking at 37°C for 7-8 hours, and inoculated into the same medium with 2% inoculation amount the next day, and continued at 37 ℃ overnight culture.
[0047] 2. vaccine preparation
[0048] Operate in accordance with the instructions of the "Gold Medal Excess Endotoxin-Free Plasmid Extraction Kit" produced by Beijing Kangwei Biotechnology Co., Ltd. Preparation and extraction of endotoxin-free plasmids—that is, nucleic acid vaccines, the specific steps are as follows:
[0049] ① Take 150ml of overnight cultured bacterial solution, add it to a centrifuge tube, centrifuge at 10,000rpm for 2-3min to collect bacteria, and discard all supernatant as much as possible.
[0050] ② Add 12ml of ...
Embodiment 3
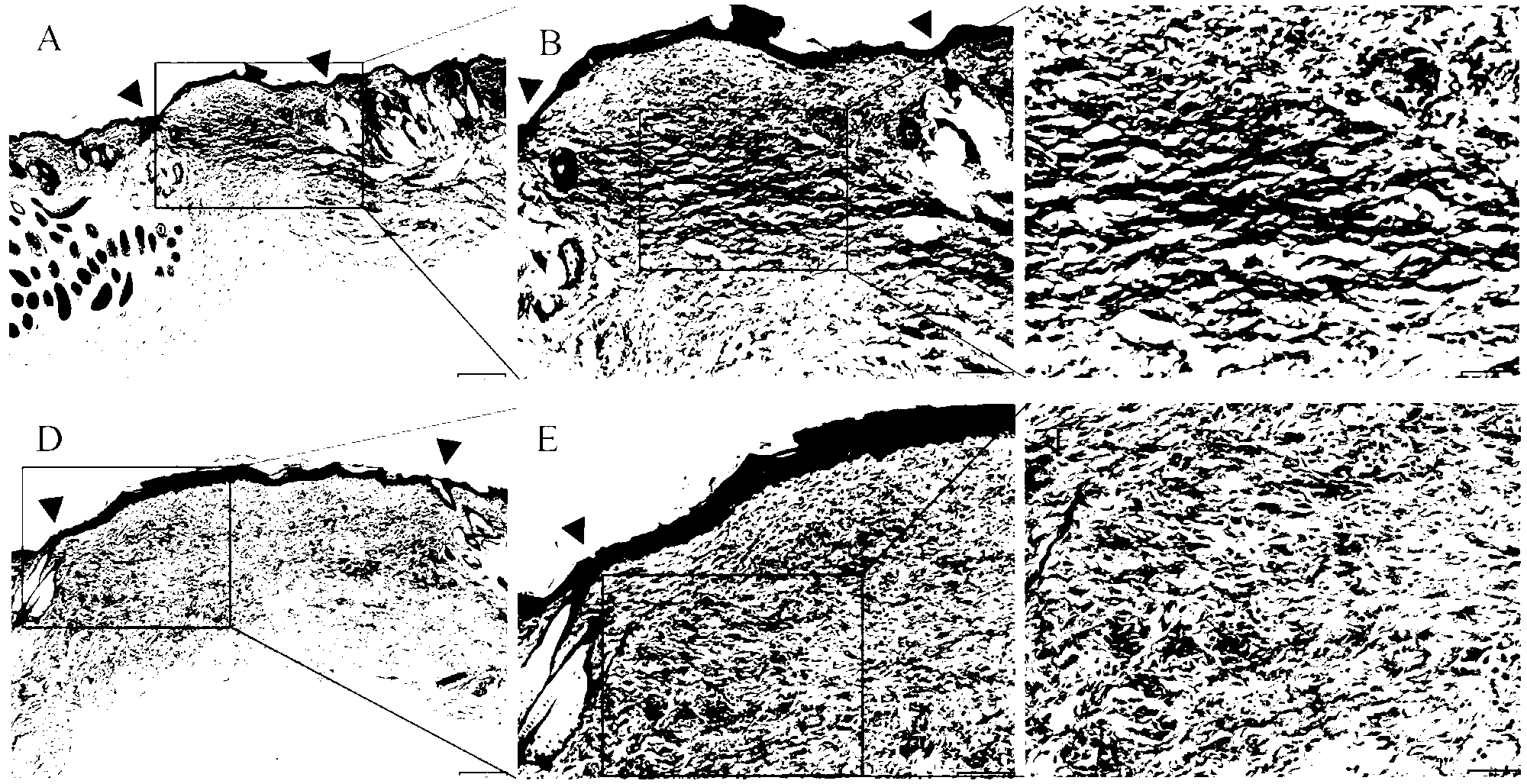
[0062] Embodiment 3: the establishment of Balb / C mouse wound healing model
[0063] (1) Establishment of wound healing model in Balb / C mice
[0064] 1. Pretreatment of Balb / C mice and injection of the first vaccine
[0065] Seven-week-old, 18 Balb / C male mice were divided into 3 groups to remove the soft hair on the back by electric clippers and powerful depilatory cream. Draw a 0.4cm×1cm rectangular area with India ink in the middle of the back of the mouse. After 24 hours, both sides of the region were subcutaneously injected: ① The blank control group was injected with 50 μl of normal saline; ② The negative control group was injected with 50 μg / 50 μl (empty vector pcDNA3. +) Empty vector; ③ The experimental group was injected with 50 μg / 50 μl (the nucleic acid vaccine pcDNA3.1-hIL10-CBD prepared by diluting with physiological saline) nucleic acid vaccine.
[0066] 2. Preparation of mouse wounds and injection of vaccine again
[0067] After 24 hours, the 0.4 cm × 1 cm ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com