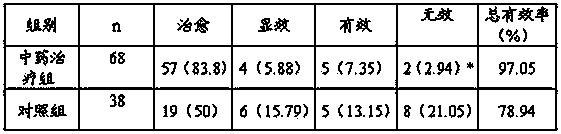
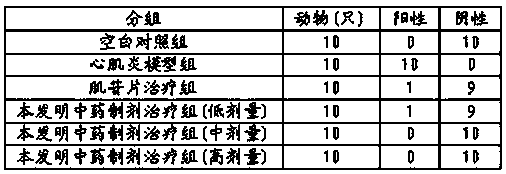
Traditional Chinese medicine preparation for treating pediatric viral myocarditis and preparation method thereof
A technology of viral myocarditis and traditional Chinese medicine preparations, which is applied in the direction of antiviral agents, anti-inflammatory agents, and pharmaceutical formulations, which can solve inconvenience and other problems, achieve high yield, reduce the amount of excipients, and reduce the clinical dose.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the dropping pill of the present invention comprises the following steps: extracting 1500 g of Pinellia chinensis and 1500 g of Earthworm by ethanol distillation to obtain crystals, then 1500 g of Ophiopogon japonicus, 1500 g of salvia miltiorrhiza, 1500 g of cassia twig, 1500 g of scallion, 1500 g of red peony root, and 1500 g of schisandra , Radix Notoginseng 1500g, Astragalus 1500g, Trichosanthes 1500g, Forsythia 1500g, Baiziren 1500g, Atractylodes 1500g, Loofah 1500g, Angelica 1500g, Chuanxiong 1500g, Curcuma 1500g, Scutellaria baicalensis 1500g, Polygala 1500g, Woody 1500g, add 5-7 Double the amount of water, put it into a multi-functional extraction tank, keep the air pressure at 0.04-0.06mPa, decoct for 2 hours, and extract its water-soluble components; repeat the above steps for another 1.5 hours, and filter the extract through a 100-mesh sieve; Adsorb the resin to refine the filtrate, and elute with ethanol; the eluate is concentrated u...
experiment example 2
[0065] Experimental example 2: Acute toxicity and long-term toxicity test results
[0066] Acute toxicity test: give drop pill of the present invention by intragastric administration of mice, maximum concentration 35%, maximum intragastric capacity 0.4ml / 10g, dosage 14g / kg (every g of medicinal powder is equivalent to 10g crude drug), observe continuously 7 days, No toxic reaction was found, and the LD 50 of the drug could not be measured because the concentration and dose could not be increased. Determination of the maximum tolerated dose: with the highest concentration and the maximum gavage volume, the mice were gavaged three times with an interval of 5 hours, and then observed continuously for 7 days, and no case died. The dosage of the medicinal powder is >42g / kg.day (each g of medicinal powder is equivalent to 10g of crude drug), which is equivalent to 420 times of the clinical daily dosage of an adult in terms of kilogram body weight. Long-term toxicity test: In order ...
experiment example 3
[0067] Experimental example 3: Cumulative toxicity test:
[0068] Traditional Chinese medicine preparation of the present invention is taken orally honey refined pills to mice by 7.69, 19.18 and 43.21g crude drug / kg continuous medication 15 weeks (1.0ml / 100g body weight, every day 2 times) and after drug withdrawal 3 weeks, the result shows: Chinese medicine of the present invention The preparation has no obvious effect on the hair, behavior, defecation, body weight, organ weight, blood picture, liver and kidney function, blood sugar, blood lipid and other indicators of rats. After 1 week and 3 weeks after stopping the drug, there was no significant change in the organs of the rats. It shows that the oral capsule of the traditional Chinese medicine preparation of the present invention has little toxicity to rats after long-term administration, and there is no abnormal reaction after drug withdrawal, so the application is safe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com