Anti-RSV (respiratory syncytial virus) human monoclonal antibody, and its preparation method
A technology of human monoclonal antibody and antibody, which is applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., and can solve the problems of high production process requirements, high price, and expensive production of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 hybridoma
[0077] 1. Mice Immunization
[0078] The cell lines expressing anti-RSV F protein were obtained by hybridoma technology screening. Add 120ul of complete Freund's adjuvant (CFA) to 120ul of RSV antigen-containing phosphate buffer solution (PBS) commercially available from Capricon, mix and emulsify to prepare 240ul of CFARSV antigen solution. In the same way, 240ul of IFA RSV antigen solution was prepared using Freund's incomplete adjuvant (IFA).
[0079] BALB / C female mice aged 7-8 weeks were intraperitoneally injected with 240ul of FCA RSV antigen solution for the first immunization. After the first immunization, booster immunization with FIA RSV antigen solution was carried out every 2-3 weeks. 240ul of RSV antigen (manufactured by Capricon) was intravenously injected 10 days and 3 days before splenocyte isolation. On the 42nd day after the immunization, the spleen was taken out, the splenocytes were separated, and the s...
Embodiment 2
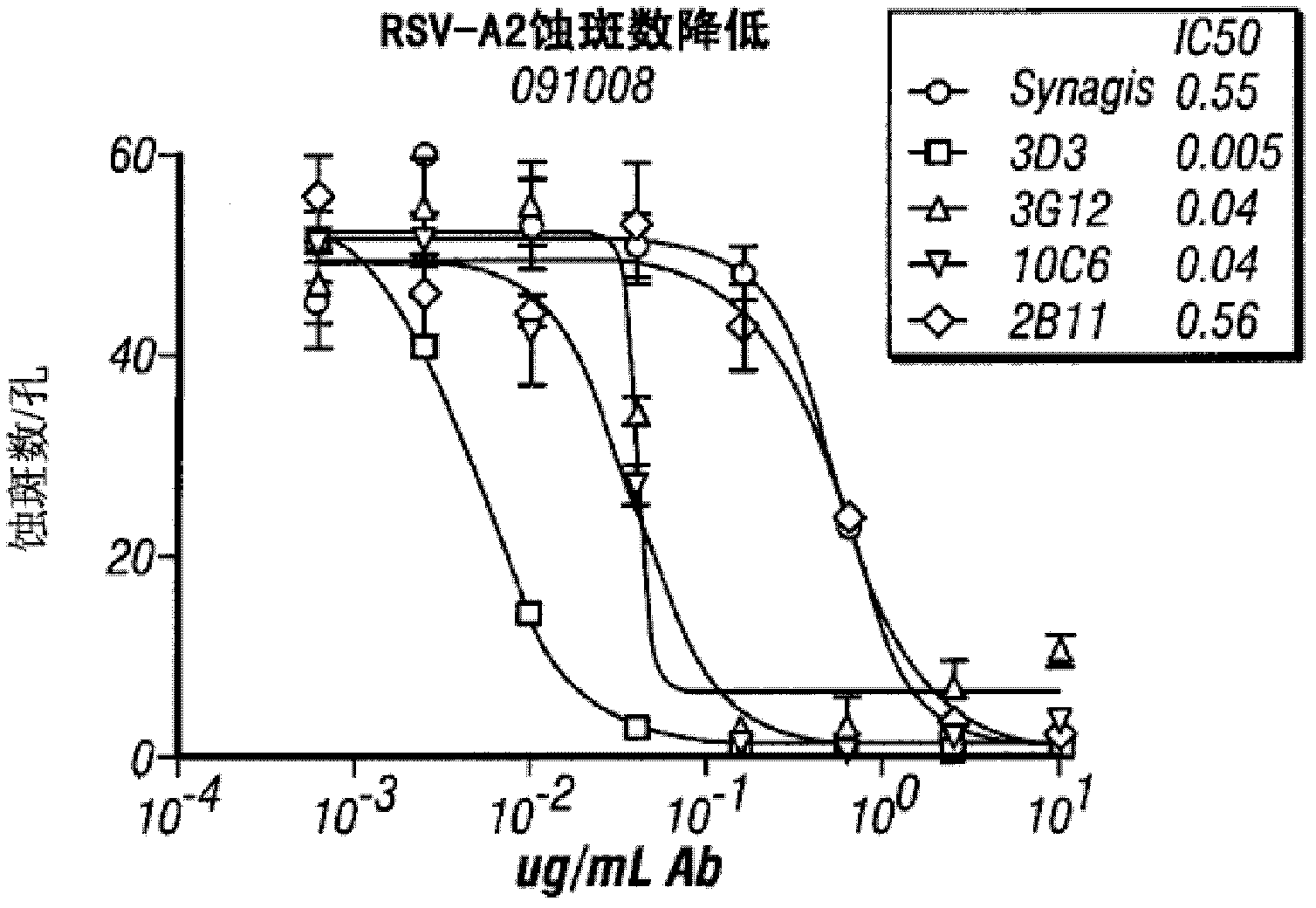
[0085] Example 2 Monoclonal Antibody Neutralizing Antigen Ability Comparison
[0086]The antigen neutralization ability of three anti-RSV monoclonal antibodies obtained by screening hybridoma cell lines was confirmed. Three RSV strains were purchased from the American Standard Biological Collection (ATCC): B1 wild-type strain (10[4.5]TCID(50) / ml), A2 (10[5.5]TCID(50) / ml) and Long Strain (10[6.7]TCID(50) / ml), the obtained RSV was diluted 10 times with phosphate buffered saline containing bovine serum albumin (BSA) (referred to as diluent).
[0087] Prepare antigen extraction reagents: 0.4M NaCl, 0.1M citric acid, 10mM dithiothreitol, and 0.1% polyoxyethylene novel phenyl ether. The diluted RSV virus and the antigen extraction reagent are mixed in equal volumes, and the antigen extraction solution is obtained after reacting for 5-10 minutes.
[0088] Prepare agarose gel (GE) coated with anti-mouse IgG polyclonal antibody (SANTA CRUZ), and the colloidal concentration is 15% (v / ...
Embodiment 37B05
[0101] Cloning and humanization of embodiment 37B05 monoclonal antibody
[0102] Follow the instructions of the RNesay kit (Qiagen) from 2 × 10 7 Total mRNA was extracted from BW01-7B05 hybridoma cells. Single-stranded cDNA was prepared using oligo-dT primers and reverse transcriptase, and an aliquot of the cDNA was used as the starting material for the polymerase chain reaction (PCR) to amplify the gene of the variable region.
[0103] The preparation of single-stranded cDNA uses 1ug of total mRNA as a template, in the buffer system of 50mM Tris-cl, 8mM Mg2Cl, 30mM KCl pH8.5, add 1mM dithiothreitol (DTT), 1mM dNTP, 25 units of human Placental ribonucleic acid inhibitor, 33uM random hexamer and 10 units of AMV reverse transcriptase, the reaction system is placed at 42°C for 1-2 hours. The heavy chain variable region (VH) of 7B05 monoclonal antibody was amplified by polymerase chain reaction (PCR) with primers P1 (SEQ ID NO:1) and P2 (SEQ ID NO:2), and the light chain variabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com