Application of erythropoietin microspheres to preparation of drugs for treating motor complications in Parkinson's disease
A technology for erythropoietin and Parkinson's disease, which is applied in the directions of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulations to achieve the effects of alleviating the pain of patients, having no toxic and side effects, and being environmentally friendly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of erythropoietin microspheres
[0028] 1. Preparation of erythropoietin polysaccharide particles
[0029] ①Use ultrapure water to prepare 10% (w / w) polyethylene glycol (PEG) aqueous solution and 10% (w / w) dextran aqueous solution respectively. Accurately weigh 800 mg of naturally extracted erythropoietin and dissolve in 7.2 ml of ultrapure water for use.
[0030] ②Under the condition of 0℃~4℃, mix the above-mentioned dextran aqueous solution, erythropoietin aqueous solution and PEG aqueous solution according to the volume ratio of 1:1:5, 1:1:10, 1:1:20 or 1:1: Mix at a ratio of 1:40, then vortex for 30s to 60s to mix thoroughly.
[0031] ③ The mixed solution of erythropoietin, PEG and dextran was frozen overnight, and then vacuum freeze-dried.
[0032] ④ Wash the sample obtained in step ③ three times with dichloromethane to remove the PEG continuous phase, and obtain erythropoietin-loaded polysaccharide particles.
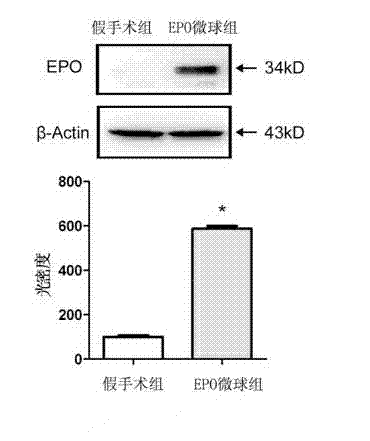
[0033] The obtained ery...
Embodiment 2
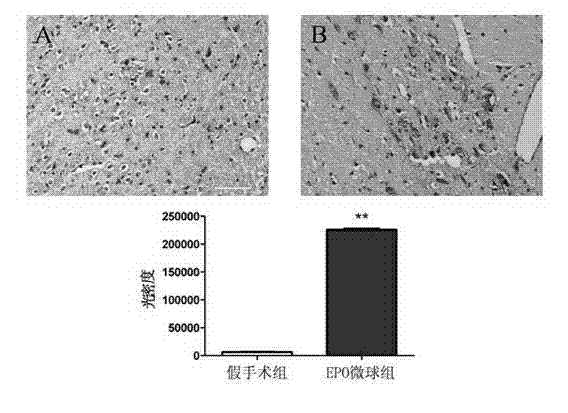
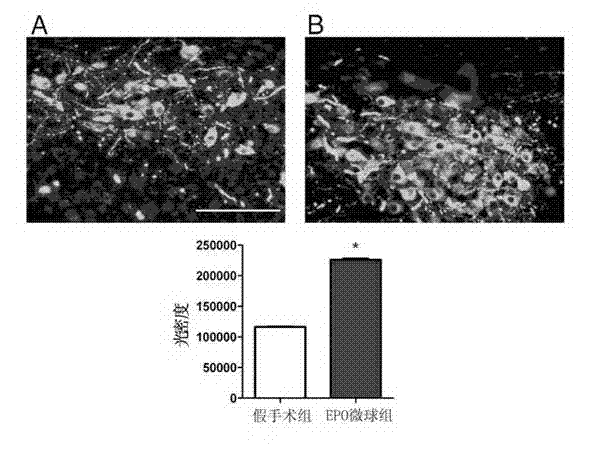
[0042] Example 2 Preclinical test of microspheres prepared from naturally extracted erythropoietin in treating motor complications of PD
[0043] 1. Experimental method
[0044] 1. Preparation of levodopa-induced dyskinesia (LID) model
[0045] After the rats were anesthetized by intraperitoneal injection of 3% pentobarbital, the cranial position was strictly fixed on the rat brain stereotaxic instrument, the skin of the head was disinfected with povidone iodine after shearing, the scalp was incised along the midline, and the periosteum was cauterized with 15% hydrogen peroxide. Expose the sutures of the skull and bregma, determine the coordinates of bregma, and determine the injection site of the right medial forebrain bundle (MFB) according to the "Rat Brain Stereotaxic Atlas" written by Bao Xinmin et al. mm, 1.7mm to the right of the sagittal suture, 8.0mm below the skull, and 2.4mm to the incisor line; ②4.4mm behind the anterior bregma, 1.2mm to the right of the sag...
Embodiment 3
[0079] Example 3 Preclinical test of microspheres prepared by gene recombination expressing erythropoietin in treating motor complications of PD
[0080] See Example 1 for the preparation method of recombinantly expressed erythropoietin microspheres, and the experimental method is the same as that of Example 2. Microspheres are prepared by gene recombinantly expressed EPO instead of naturally extracted EPO, injected subcutaneously or intracranially into LID rats, and then detected Corresponding indicators. Table 2 shows the effect of the microspheres prepared by genetically recombinantly expressing EPO on the number of rotations of the rat model.
[0081] Table 2 The effect of different doses of gene recombinant expression EPO microspheres on the number of rotations of the rat model
[0082]
[0083] It can be seen from the table that the microspheres prepared by gene recombination expressing EPO can reduce the number of involuntary rotations of LID rats, and high-dose E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com