Cadmium ion fluorescence probe, preparation method and application
A technology of fluorescent probes and cadmium ions, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as poor water solubility, ultraviolet excitation, and low complexing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] a. Dissolve intermediate I as 8-oxoethyl quinoline-2-carbaldehyde (25.9 mg, 0.1 mmol) and heterocrown ether (25.1 mg, 0.1 mmol) in 1,2-dichloroethyl ether at room temperature alkane (10 mL), stirred for 10 minutes, and then added NaBH(OAc) in batches 3 (0.22 g, 0.11 mmol), react overnight at room temperature;
[0025] b. After the reaction, acidify with 1N hydrochloric acid to pH = 4-5, then neutralize with 1N sodium hydroxide solution to pH = 7-8, then extract 3 times with ethyl acetate, collect the organic phase, and use anhydrous Dry over sodium sulfate, distill off the solvent under reduced pressure, and purify with silica gel chromatography (dichloromethane / 25% ethyl acetate) to obtain intermediate II as 2-methylene-(1,4-dioxo-7,13 disulfide -Aza15crown5)-(quinoline-8-oxyl)ethyl acetate, yield 46%;
[0026] c. Dissolve the intermediate II (0.45 g, 1.0 mmol) obtained in step b in ethanol (5 mL), add 10 mg NaOH, and stir overnight at room temperature;
[0027] d. ...
Embodiment 2
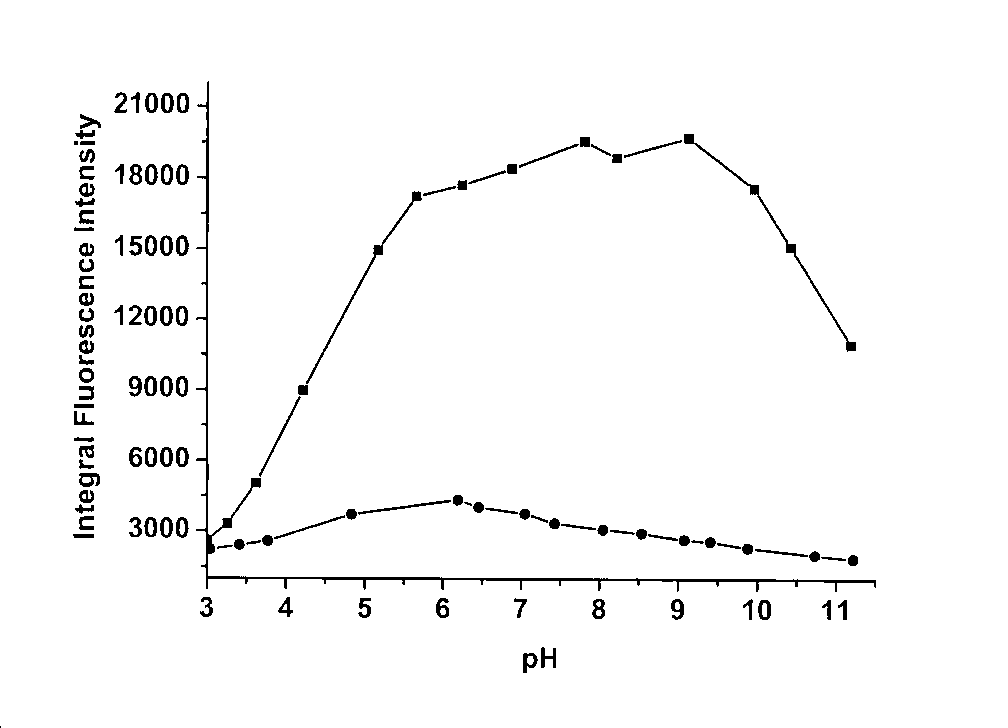
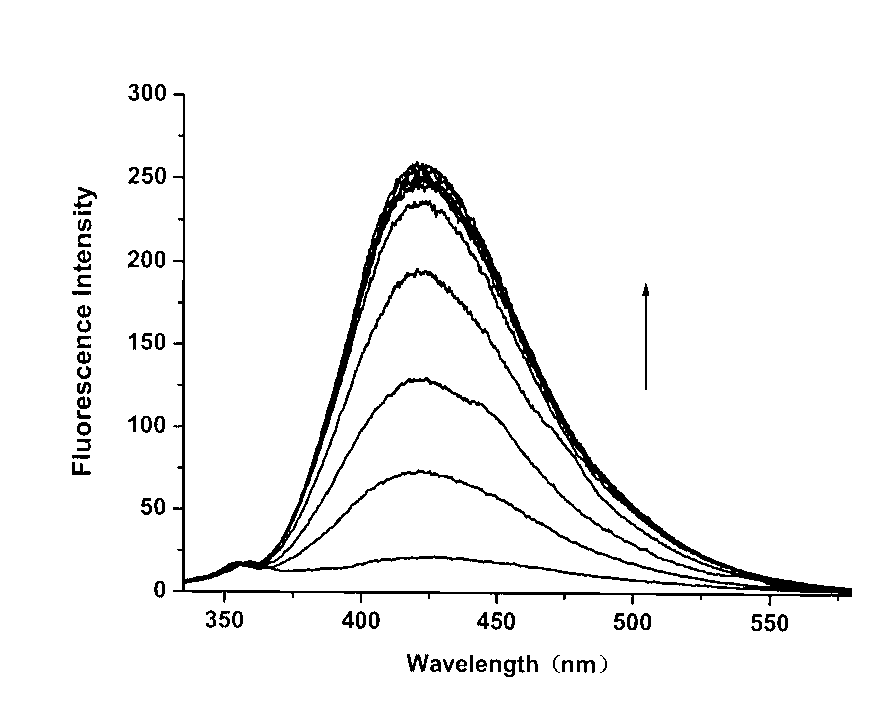
[0030]In the buffer solution (25 mM HEPES, 0.1 M NaClO4, pH = 7.4), the concentration of the fluorescent probe was 5 μM, and the concentration of cadmium ion was 0, 1.25, 2.5, 3.75, 5, 6, 8, 10, 20 μM, and the excitation The wavelength is 310nm, and the slit width is 5nm\5nm;
[0031] In buffer solution (25 mM HEPES, 0.1 M NaClO 4 , pH = 7.4), the maximum absorption peak of the fluorescent probe itself is at 241 nm, and there is a broad absorption band at 270-330 nm in the long wavelength direction, when Cd is added to the solution 2+ (0~1.5 equiv), the 241 nm absorption peak gradually weakened and slightly red-shifted, while due to the Cd 2+ Coordination with quinoline, the absorption band at long wavelengths is slightly red-shifted to 300-350 nm; at the same time, after adding Cd 2+ After that, they produced three isosbestic points at 245 nm, 257 nm, and 310 nm, which indicated that only one cadmium ion complex was formed in the solution, and when 1.0 equivalent of Cd was ...
Embodiment 3
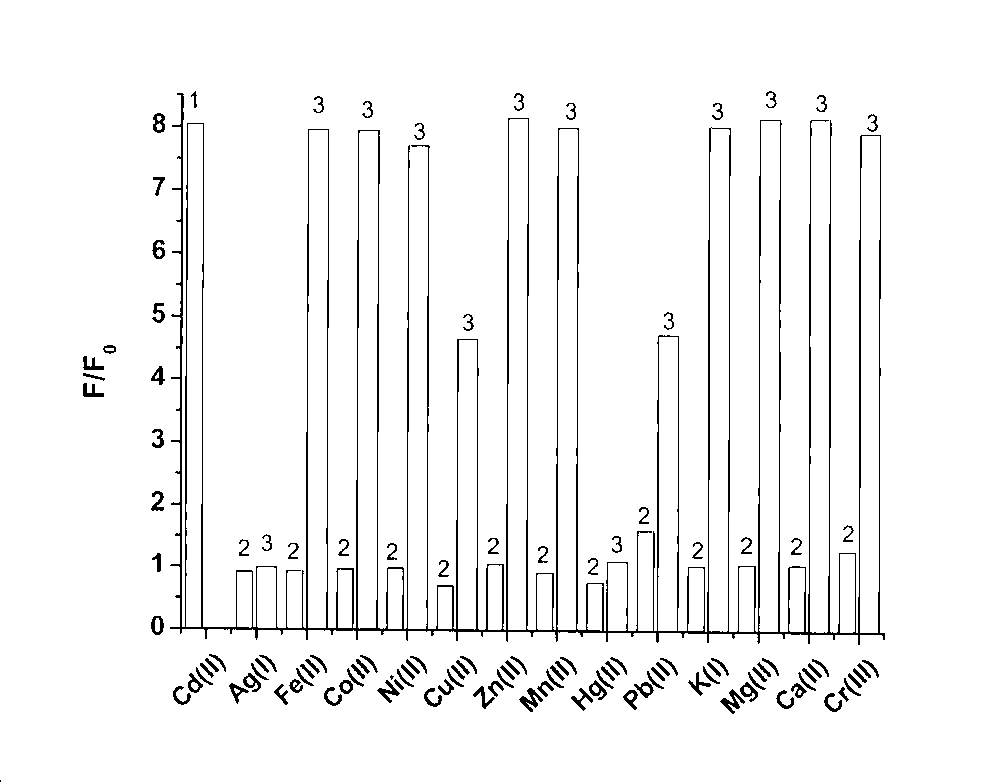
[0035] Prepare a 5 μM fluorescent probe solution, and then add equivalent amounts of different metal ions Ag to the solution + 、K + , Ca 2+ , Mg 2+ , Mn 2+ , Fe 2+ 、Co 2+ 、Ni 2+ 、Cu 2+ , Zn 2+ 、Cd 2+ , Hg 2+ , Pb 2+ , to measure the fluorescence emission spectrum of the fluorescent probe molecule in the presence of each metal ion;
[0036] With reference to the fluorescence spectrometry titration experiment of cadmium ions on probe molecules, the present invention judges the selectivity of probes to cadmium ions by competitive metal ion titration experiments, and adds equivalent amounts of Ag respectively in the solution. + , Mn 2+ , Fe 2+ 、Co 2+ 、Ni 2+ 、Cu 2+ , Zn 2+ , Hg 2+ , Pb 2+ and 100 equivalents of K + , Ca 2+ , Mg 2+ After that, the fluorescence spectrum did not change significantly, only the addition of Cd 2+ Afterwards, the fluorescence spectrum has just produced very big enhancement, shows by the ratio F / F0 monitoring of fluorescence emission...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com