Method for detecting blood homocysteine and kit
A homocysteine, detection kit technology, applied in the measurement of color/spectral properties, material analysis by observing the effect on chemical indicators, material excitation analysis, etc., can solve the problem of high sensitivity, high price, color development Reaction and other problems, to achieve the effects of good reagent stability, sensitive method and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
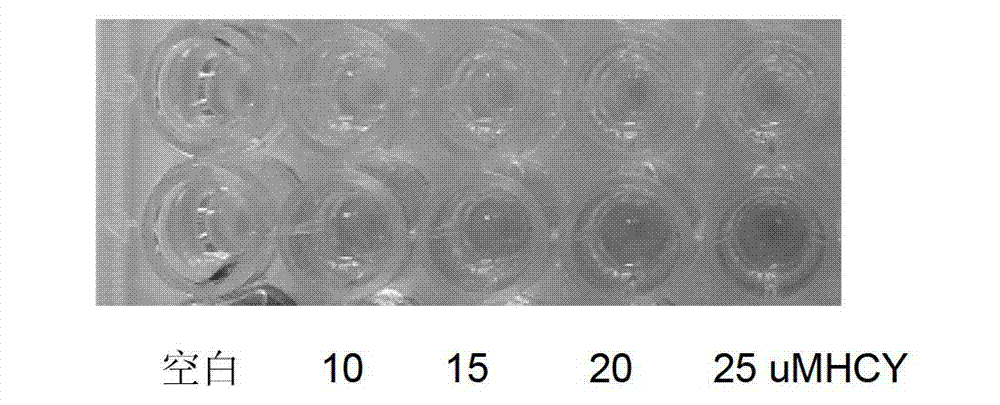
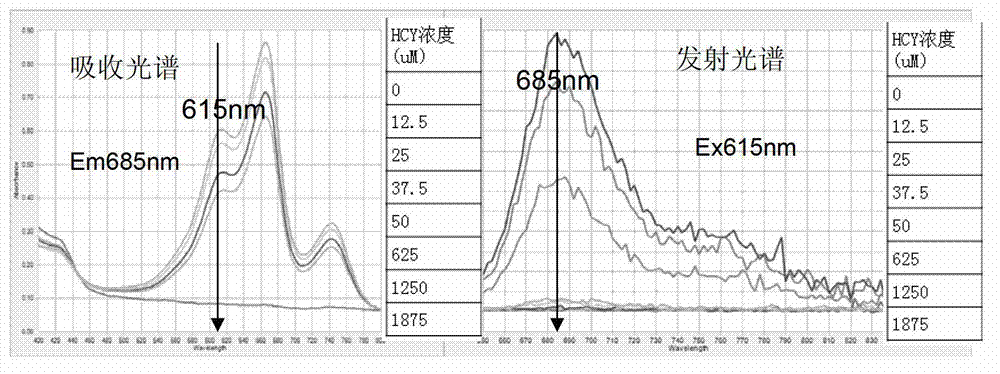
[0031] HCY generates H under the catalysis of recombinant rHCYase 2 S, H 2 S and dimethyl-p-aniline in F 3+ Under oxidation, the reaction produces blue methyl blue. The depth of blue is proportional to the concentration of HCY. The darker the color, the higher the concentration of HCY. Based on this principle, the kit is prepared, such as figure 1Shown is the color chart of HCY detection. Add HCY and cysteine at concentrations of 0, 10, 15, 20, and 25 μM to the well plate, and perform detection according to the color detection method. There is no obvious cysteine in the upper row. Color, the lower row of color, with the increase of HCY concentration, the darker the color, the absorbance is proportional to the concentration of HCY.
[0032] Fluorescence detection kit consists of: a 96-well blackboard, 27.03 μg of HCY standard substance, 3 mg of HCY-specific converting enzyme, 50 ml of diluent, 0.771 μg of reducing agent, 10 ml of chromogenic solution A, and 10 ml of chrom...
Embodiment 2
[0042] The composition of the colorimetric detection kit: a 96-well transparent microplate, HCY standard 27.03μg, HCY-specific converting enzyme 2mg, diluent 50ml, reducing agent 0.771μg, chromogenic solution A10ml, chromogenic solution B10ml.
[0043] Steps:
[0044] 1) Prepare HCY standard substance with diluent to make 1ml solution, the concentration is 200μmol / L, after serial doubling dilution, dilute to 100μmol / L, 50μmol / L, 25μmol / L, 10μmol / L, 5μmol / L L, 2.5μmol / L, 0μmol / / L, the diluent is directly used as the standard concentration of 0μmol / / L, and it is prepared within 15 minutes before use. Dissolve the reducing agent with 5ml of diluent.
[0045] 2) Add 100 μl of HCY standard and sample to a 96-well transparent microtiter plate, and then add 10 μl of reducing agent to reduce for 1 h;
[0046] 3) Dissolve HCY-specific invertase with 5ml diluent, then add 20 μl of HCY-specific invertase solution to the above reaction solution for 5 minutes;
[0047] 4) Add 50 μl of c...
Embodiment 3
[0051] The HCY of the serum sample is measured by the detection method of the present invention, and the result is compared with the measurement result of high performance liquid chromatography (HPLC).
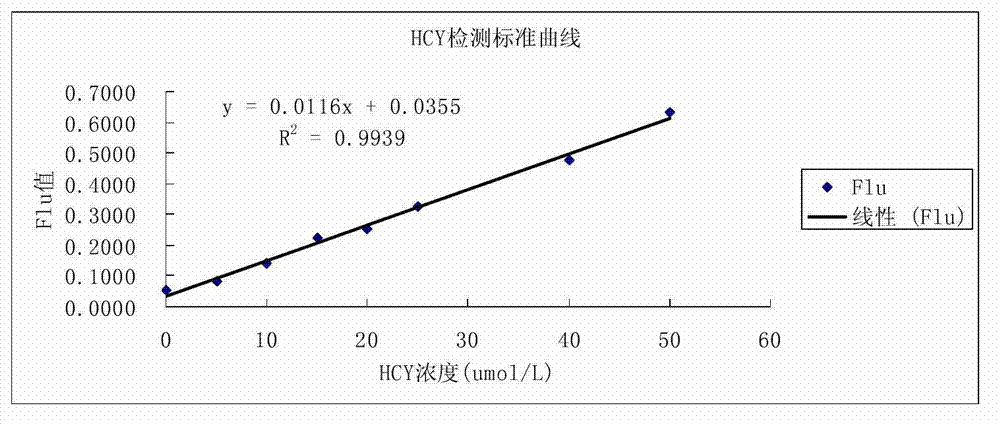
[0052] Select 10 parts of serum samples measured by HPLC method, measure with the method of the present invention, measure the result and compare with HPLC method measure result, the result is as shown in Table 1, and the calculated coincidence rate is 0.9998. It shows that the result of this method is reliable.
[0053] Table 1 comparison of measurement results
[0054]
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com