Brown coloring method for high borosilicate glass
A high borosilicate glass, brown technology, applied in glass surface treatment, ion diffusion in the field of high borosilicate glass surface coloring, can solve the problems of cumbersome process, difficult control, high production cost, etc., to achieve simple preparation process, smooth surface, The effect of short processing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
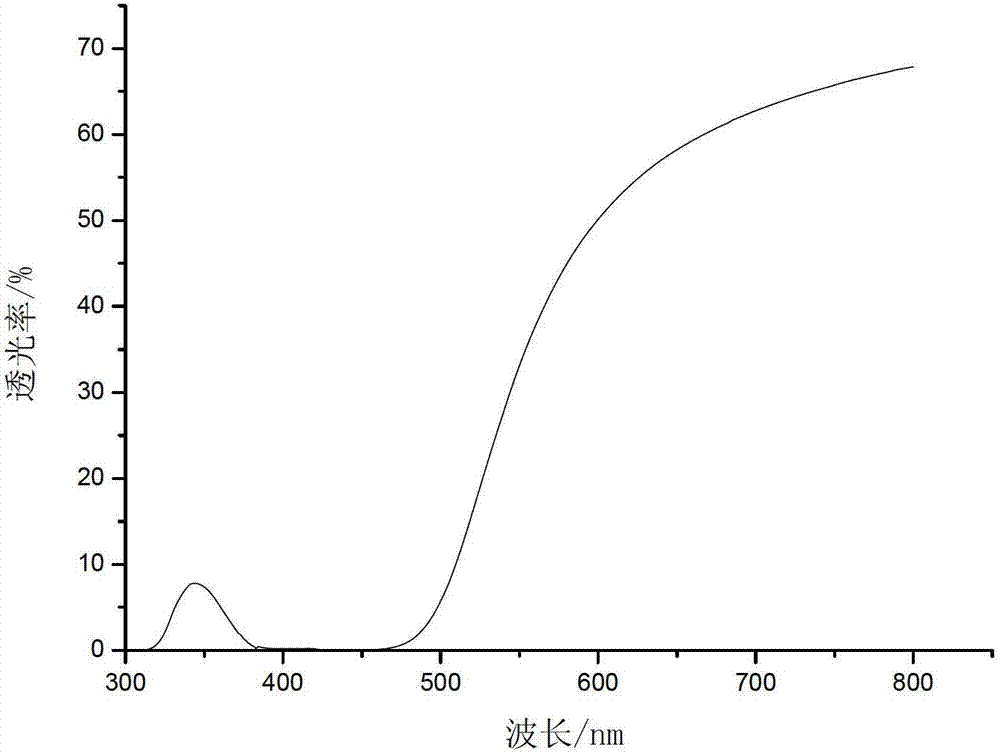
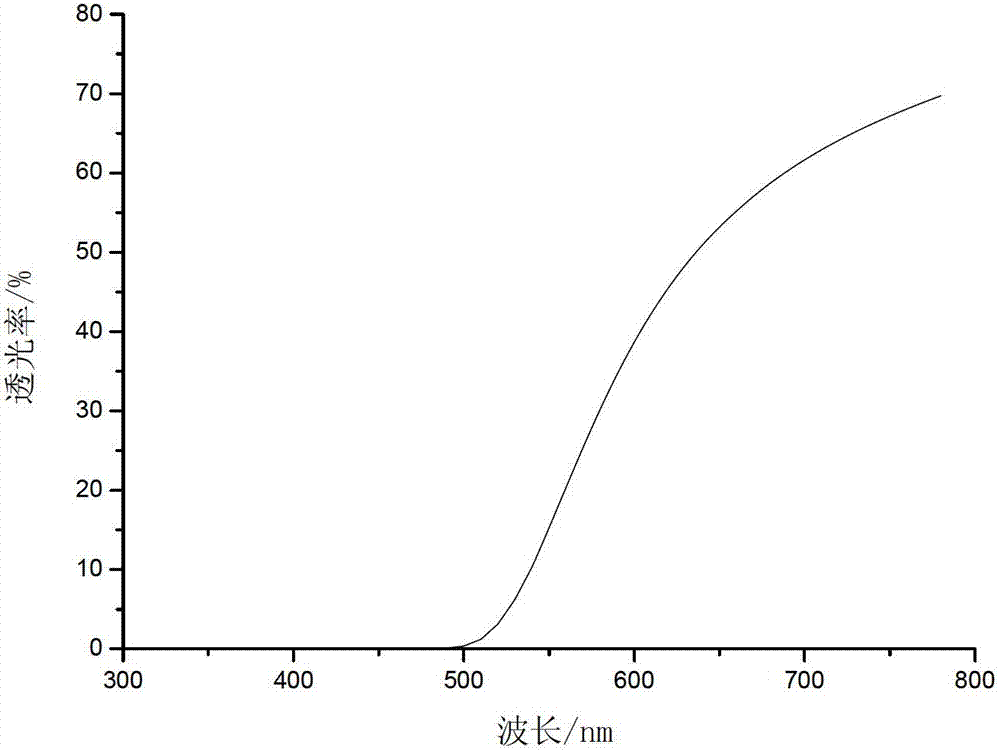
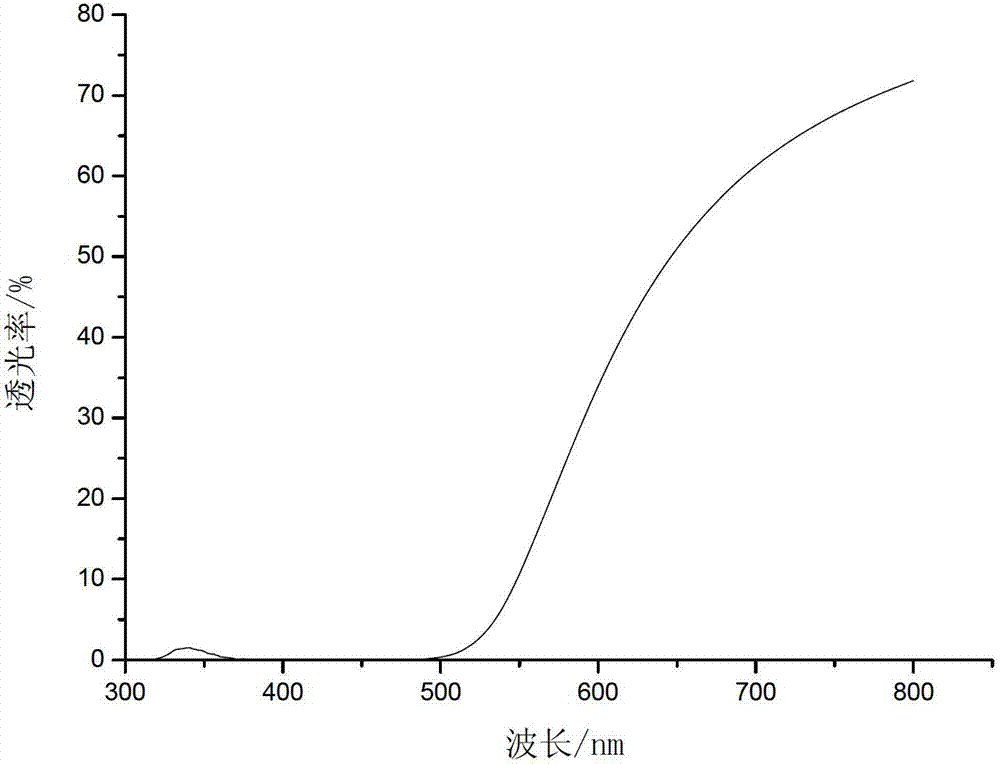
Image
Examples
Embodiment 1
[0039] (1) Preparation of colorant paste:
[0040]① Weigh raw materials according to the following components and parts by mass:
[0041] Dextrin powder
7
NaOH
4
AgNO 3
10
CuSO 4
42
FeSO 4
8
toner
13
Zinc powder
3
Glycerin
4
water
12
[0042] ② Preparation of coloring paste
[0043] First, dissolve NaOH in deionized water to prepare NaOH solution, use NaOH solution to reconcile the dextrin powder into a paste (no agglomeration and air bubbles are allowed); then add analytically pure AgNO 3 , CuSO 4 , FeSO 4 , carbon powder and zinc powder, stir evenly, add glycerin after cooling and continue stirring to obtain a mixed slurry.
[0044] (2) Pour the mixed slurry obtained in step ② into a ball mill tank and mill for 3 hours to prepare a coloring material paste whose particle size is required to pass through a 200-mesh sieve.
[0045] (3) Apply ...
Embodiment 2
[0049] (1) Preparation of colorant paste:
[0050] ① Weigh raw materials according to the following components and parts by mass:
[0051] Dextrin powder
10
NaOH
7
AgNO 3
9
CuSO 4
45
FeSO 4
7
toner
5
Zinc powder
4
Glycerin
4
water
15
[0052] ② Preparation of coloring paste
[0053] First, dissolve NaOH in deionized water to prepare NaOH solution, use NaOH solution to reconcile the dextrin powder into a paste (no agglomeration and air bubbles are allowed); then add analytically pure AgNO 3 , CuSO 4 , FeSO 4 , carbon powder and zinc powder, stir evenly, add glycerin after cooling and continue stirring to obtain a mixed slurry.
[0054] (2) Pour the mixed slurry obtained in step ② into a ball mill tank and mill for 4 hours to prepare a coloring material paste, and the particle size is required to pass through a 300-mesh sieve.
[0055] (3) Use...
Embodiment 3
[0059] (1) Preparation of colorant paste:
[0060] ① Weigh raw materials according to the following components and parts by mass:
[0061] Dextrin powder
4
NaOH
7
AgNO 3
11
CuSO 4
58
FeSO 4
12
toner
10
Zinc powder
5
Glycerin
3
water
9
[0062] ② Preparation of coloring paste
[0063] First, dissolve NaOH in deionized water to prepare NaOH solution, use NaOH solution to reconcile the dextrin powder into a paste (no agglomeration and air bubbles are allowed); then add analytically pure AgNO 3 , CuSO 4 , FeSO 4 , carbon powder and zinc powder, stir evenly, add glycerin after cooling and continue stirring to obtain a mixed slurry.
[0064] (2) Pour the mixed slurry obtained in step ② into a ball mill tank and mill for 3 hours to prepare a coloring material paste whose particle size is required to pass through a 250-mesh sieve.
[0065] (3) Apply...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com