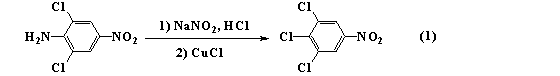
Method for preparing 2,6-dichloro-alpha-(4-chlorphenyl)-4-nitro phenylacetonitrile
A technology of nitrophenylacetonitrile and chlorophenylacetonitrile, which is applied in the field of preparation of anticoccidial intermediate 2,6-dichloro-α--4-nitrophenylacetonitrile, which can solve the problem of 4-position chlorine being substituted Low cost, difficult to complete condensation, high cost, etc., to achieve high industrial production value, avoid isomers, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Get 50g of distillation residue of 2,4-dichloro-3-fluoronitrobenzene production process (3,5-dichloro-4-fluoronitrobenzene content 87.7%, 2,4-dichloro-3-fluoronitrobenzene The content of benzene is 9.6%, and the content of other impurities is 2.3%. It comes from a dark brown distillation residue with batch number LH20111223 of an enterprise in Linhai, Zhejiang Province in 2011) and is heated and dissolved in 200mL of petroleum ether. Remove the solvent, recrystallize with methanol, filter, wash with cold methanol, and dry in vacuo to obtain 3,5-dichloro-4-fluoronitrobenzene with a content of 98.3% and a yield of 77%.
[0025] Take 21.0g (0.1mol) of 3,5-dichloro-4-fluoronitrobenzene obtained above, 70g of sodium hydroxide dissolved in 70mL of water to make 50% liquid caustic soda, 3g of benzyltriethylammonium chloride and 140mL In tetrahydrofuran, add dropwise a solution of 17.0g p-chlorophenylacetonitrile in 50mL THF at 30°C, heat up to 50°C for 6 hours after dropping, ...
Embodiment 2
[0027]Take 2,4-dichloro-3-fluoronitrobenzene distillation residue 50g (3,5-dichloro-4-fluoronitrobenzene content 86.5%, 2,4-dichloro-3-fluoronitrobenzene The content of benzene is 10.1%, and the content of other impurities is 1.2%, which is derived from the taupe distillation residue with batch number KH20120211 of a company in Kaihua, Zhejiang Province in 2012). The solvent was distilled off, recrystallized with absolute ethanol, filtered, washed with cold ethanol, and dried in vacuum to obtain 3,5-dichloro-4-fluoronitrobenzene with a content of 98.4% and a yield of 75%.
[0028] Take 21.0g (0.1mol) of 3,5-dichloro-4-fluoronitrobenzene obtained as above, 50% liquid caustic soda prepared by dissolving 60g of sodium hydroxide in 60mL of water, 3g of tetrabutylammonium bromide and 120mL of tetrahydrofuran, Add dropwise a solution of 17.0g p-chlorophenylacetonitrile in 50mL tetrahydrofuran at 40°C, heat up to 60°C for 4 hours after dropping, pour the mixture into crushed ice, aci...
Embodiment 3
[0030] Get 50g of distillation residue of 2,4-dichloro-3-fluoronitrobenzene production process (3,5-dichloro-4-fluoronitrobenzene content 84.3%, 2,4-dichloro-3-fluoronitrobenzene The content of benzene is 12.4%, and the content of other impurities is 2.7%. It comes from a dark brown distillation residue with batch number QZ20120324 of a company in Quzhou, Zhejiang Province in 2012) and is heated and dissolved in 260mL of toluene. The solvent was recrystallized once with isopropanol and methanol respectively, and vacuum-dried to obtain 3,5-dichloro-4-fluoronitrobenzene with a content of 98.5% and a yield of 72%.
[0031] Take 21.0g (0.1mol) of 3,5-dichloro-4-fluoronitrobenzene obtained above, 70g of sodium hydroxide dissolved in 70mL of water to make 50% liquid caustic soda, 3g of benzyltriethylammonium chloride and 140mL Add dropwise a solution of 17.0g p-chlorophenylacetonitrile in 50mL tetrahydrofuran to THF at 30°C, heat up to 50°C and react for 6h after dropping, pour the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com