Preparation method of tert-butyl carbazate
A technology of carbazic acid and tert-butyl ester, which is applied in the field of one-pot preparation of tert-butyl carbazate, which can solve the problems of long reaction time, difficulty in industrialization, and expensive medicines, and achieve simple process and easy operation , the effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
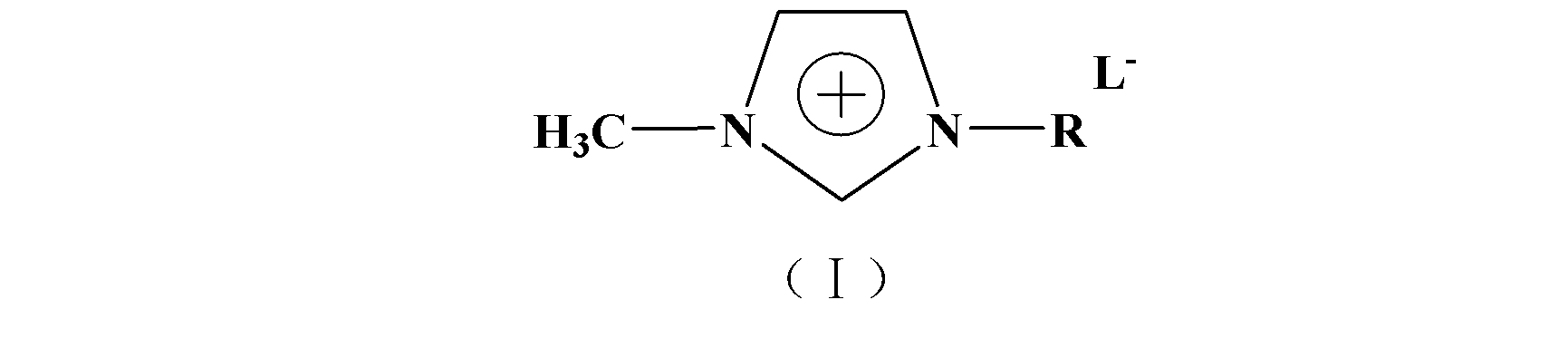
[0023] 15.7 grams (0.1 moles) of phenyl chloroformate, 8 grams (0.1 moles) of tert-butanol, 1.5 grams of solid base catalyst prepared by the method of Example 1 and 1-methyl-3-butylimidazolium tetrafluoroborate ion Add 3 grams of liquid into the reactor, and after esterification at 30°C for 6 hours, add 64 grams (0.1 mole) of hydrazine hydrate aqueous solution with a mass concentration of 50% (0.1 mole) for 3 hours of replacement reaction at 60°C. After the reaction, cool the reaction solution to At room temperature (25°C), add ethyl acetate (3×50ml) for extraction (i.e. extract 3 times, 50mL each time, the same below), take the extract and concentrate under reduced pressure until no liquid flows out to remove the extractant, and the obtained concentrate Carry out silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 (volume ratio)), TLC follow-up detection, collect the eluate containing the target component, and concentrate to dryness under reduced pressure to...
Embodiment 2
[0025]15.7 grams (0.1 moles) of phenyl chloroformate, 24 grams (0.3 moles) of tert-butanol, 3 grams of solid base catalyst prepared by the method in Example 1 and 1-methyl-3-butylimidazole hexafluorophosphate ionic liquid Add 2.5 grams into the reactor, and after esterification at 30°C for 1 hour, add 96 grams (0.15 moles) of hydrazine hydrate aqueous solution with a mass concentration of 50% (0.15 moles) for 1 hour of substitution reaction at 60°C. After the reaction, cool the reaction solution to At room temperature (25°C), add ethyl acetate (3×50ml) for extraction (i.e. extract 3 times, 50mL each time, the same below), take the extract and concentrate under reduced pressure until no liquid flows out to remove the extractant, and the obtained concentrate Carry out silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 (volume ratio)), TLC follow-up detection, collect the eluate containing the target component, and concentrate to dryness under reduced pressure ...
Embodiment 3
[0027] 15.7 grams (0.1 moles) of phenyl chloroformate, 5 grams (0.3 moles) of tert-butanol, 5 grams of solid base catalyst prepared by the method in Example 1 and 1-methyl-3-octylimidazolium tetrafluoroborate ion Add 4 grams of liquid into the reactor, and after esterification reaction at 40°C for 1 hour, add 128 grams (0.2 moles) of hydrazine hydrate aqueous solution with a mass concentration of 50% (0.2 moles) to replace and react at 75°C for 5 hours. After the reaction, cool the reaction liquid to room temperature (25°C), add ethyl acetate (3×50ml) for extraction (i.e. extract 3 times, 50mL each time, the same below), take the extract and concentrate under reduced pressure until no liquid flows out to remove the extractant, and the obtained concentrated The liquid was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 (volume ratio)), followed by TLC detection, and the eluate containing the target component was collected and concentrated to d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com