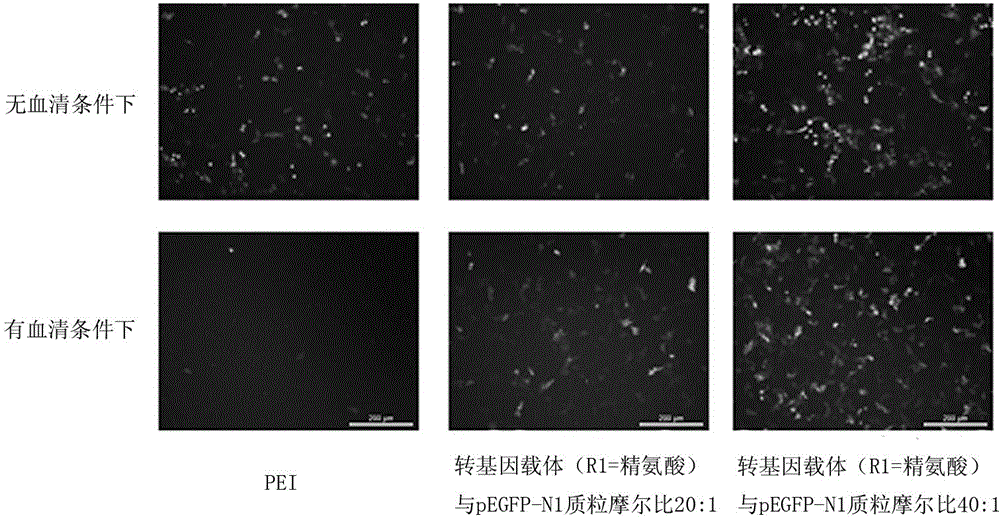
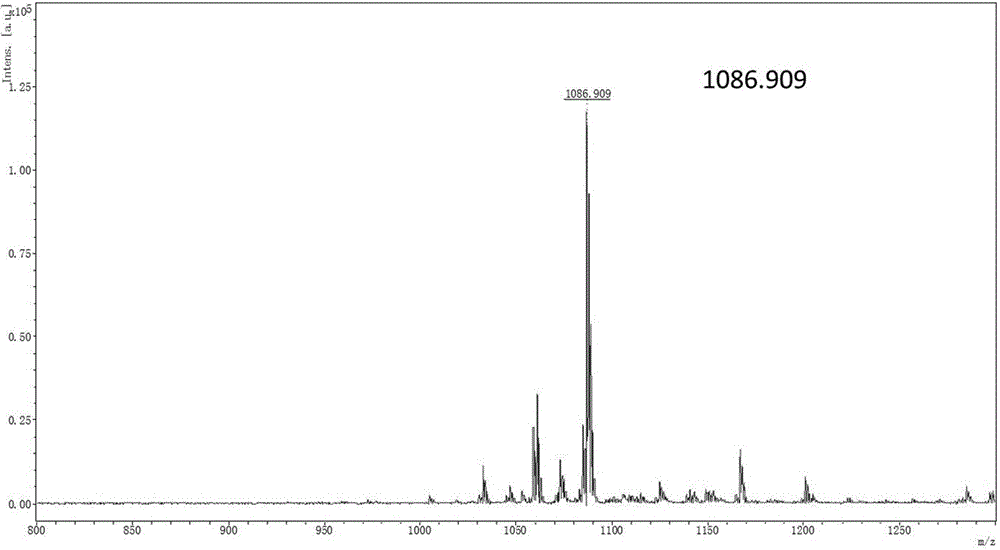
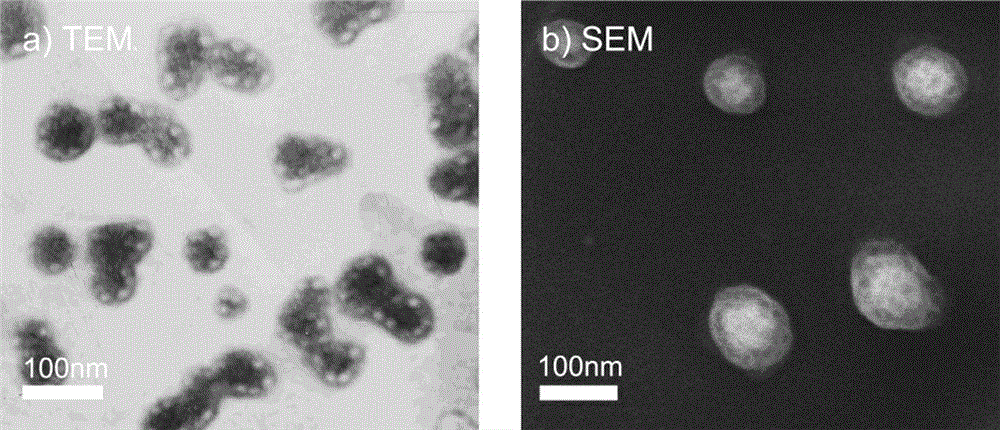
Cationic lipid containing peptide dendrimer, transgenic carrier and preparation method and application of transgenic carrier
A peptide dendrimer, cationic lipid technology, applied in the direction of introducing foreign genetic material, peptides, recombinant DNA technology using vectors, etc., can solve the problems of host DNA insertion and integration, low transfection efficiency, carcinogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] The raw materials include 1 mmol, 1.20 g of compound 1 (peptide dendrimer protected by the second-generation amino Pbf / Boc, R1=arginine), 1 mmol, 0.66 g of compound 2 (fatty alkane amide of glutamic acid), condensing agent (such as: 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride EDC, 2mmol, 0.38g, catalyst 1-hydroxybenzotriazole (HOBt, 2mmol, 0.26g), base (N , N-diisopropylethylamine DIPEA, 4mmol, 0.5g), the solvent is anhydrous dichloromethane, compound 1: compound 2: condensing agent: catalyst: base (molar ratio) =1: 1: 2: 2: 4. The ratio is a molar ratio, and the amount of solvent is limited to the complete dissolution of compound 1, compound 2, condensing agent, catalyst, and alkali;
[0039] Weigh compound 1, compound 2, condensing agent, 1-hydroxybenzotriazole (HOBt) and base in proportion, at 0 o C, under nitrogen protection conditions, add dichloromethane solvent, react for 0.5 hours; then react at room temperature for 24 hours, after the...
Embodiment 2
[0047]
[0048] The raw materials include compound 1 (second-generation amino Pbf / Boc protected peptide dendrimer, R1=arginine) 1mmol, 1.20 g, compound 4 (N1-cholesterolamide-1,2-ethylenediamine) 1mmol, 0.47 g , condensing agent (such as: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride EDC·HCl, 2mmol, 0.38g, catalyst 1-hydroxybenzotriazole (HOBt, 2mmol , 0.26g), base (N, N-diisopropylethylamine DIPEA, 4mmol, 0.5g), solvent is anhydrous dichloromethane, compound 1: compound 4: condensing agent: catalyst: base=1: 1: 2: 2: 4, described ratio is molar ratio, and the amount of solvent is limited with compound 1, compound 4, condensing agent, catalyzer, alkali completely dissolving;
[0049] Weigh compound 1, compound 4, condensing agent, catalyst (1-hydroxybenzotriazole, HOBt) and base in proportion, at 0 o C, under nitrogen protection conditions, add dichloromethane solvent, react for 0.5 hours; then react at room temperature for 24 hours, after the reaction is over...
Embodiment 3
[0052] Example 3. The preparation of the transgenic carrier containing cationic lipid in example 1
[0053] The raw materials include the cationic lipid containing peptide dendrimers prepared in Example 1, cholesterol and anhydrous chloroform, 0.01 mmol of cationic lipid, 0.01 mmol of cholesterol, and 0.5 mL of anhydrous chloroform. Add the cationic lipid, cholesterol and anhydrous chloroform into the eggplant-shaped bottle at room temperature (25°C) and normal pressure. After the cationic lipid and cholesterol are completely dissolved, use a rotary evaporator (100 r / min) The chloroform was distilled off to obtain a dry lipid film, which was then dried in vacuo overnight to remove residual chloroform. Add high-purity sterilized water to the dried lipid film to make a 4 mmol / L solution, stir for 1 hour, ultrasonicate the probe for 1 hour, and finally use liposoFast TM The transgenic vector containing cationic lipids was prepared by liposome extruder, and stored in a refrige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com