Novel EP4 agonist
A technology of agonists and pharmaceuticals, applied in the direction of anti-inflammatory agents, non-central analgesics, digestive system, etc., can solve the problems of weak binding affinity and only strong binding affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
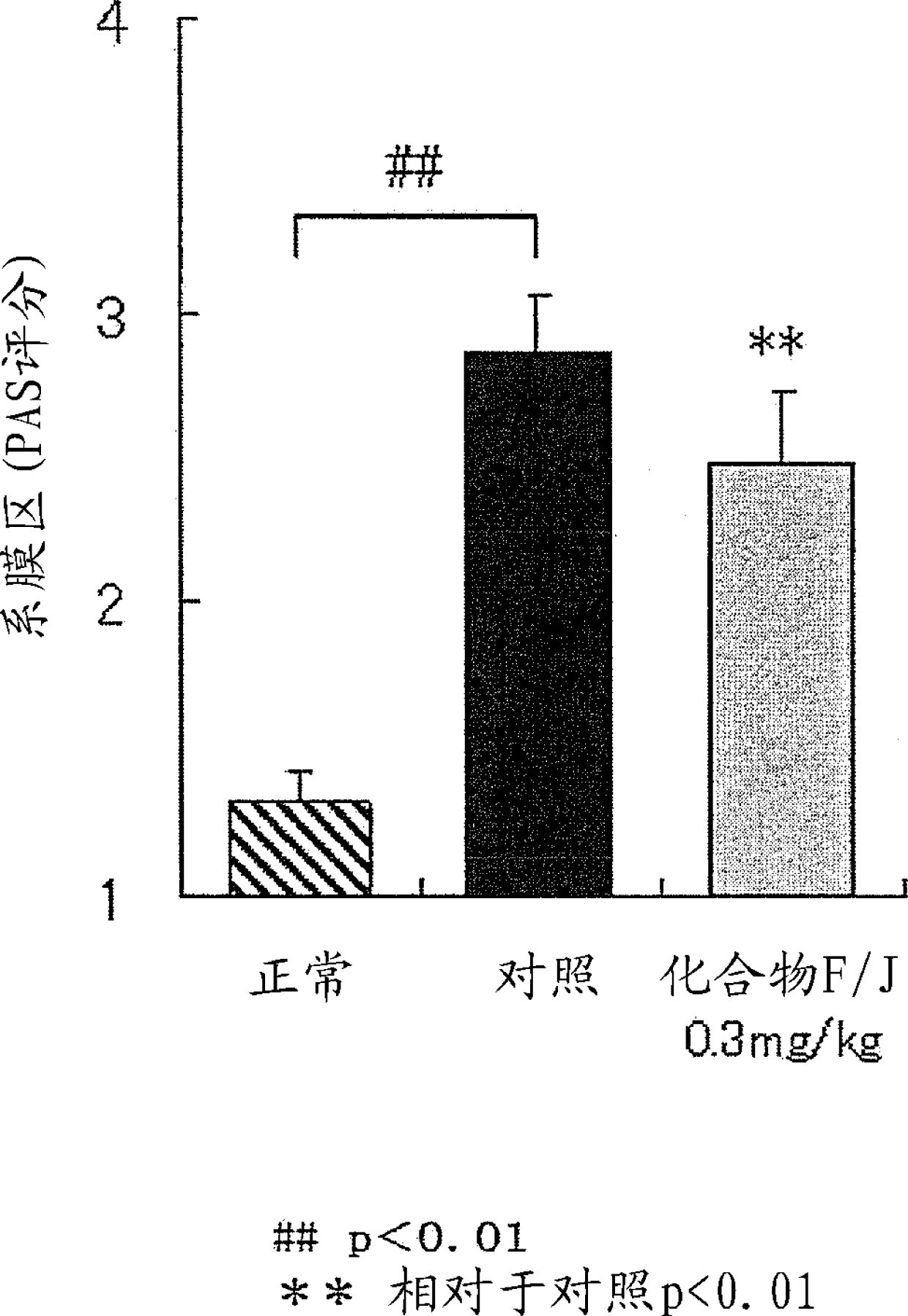
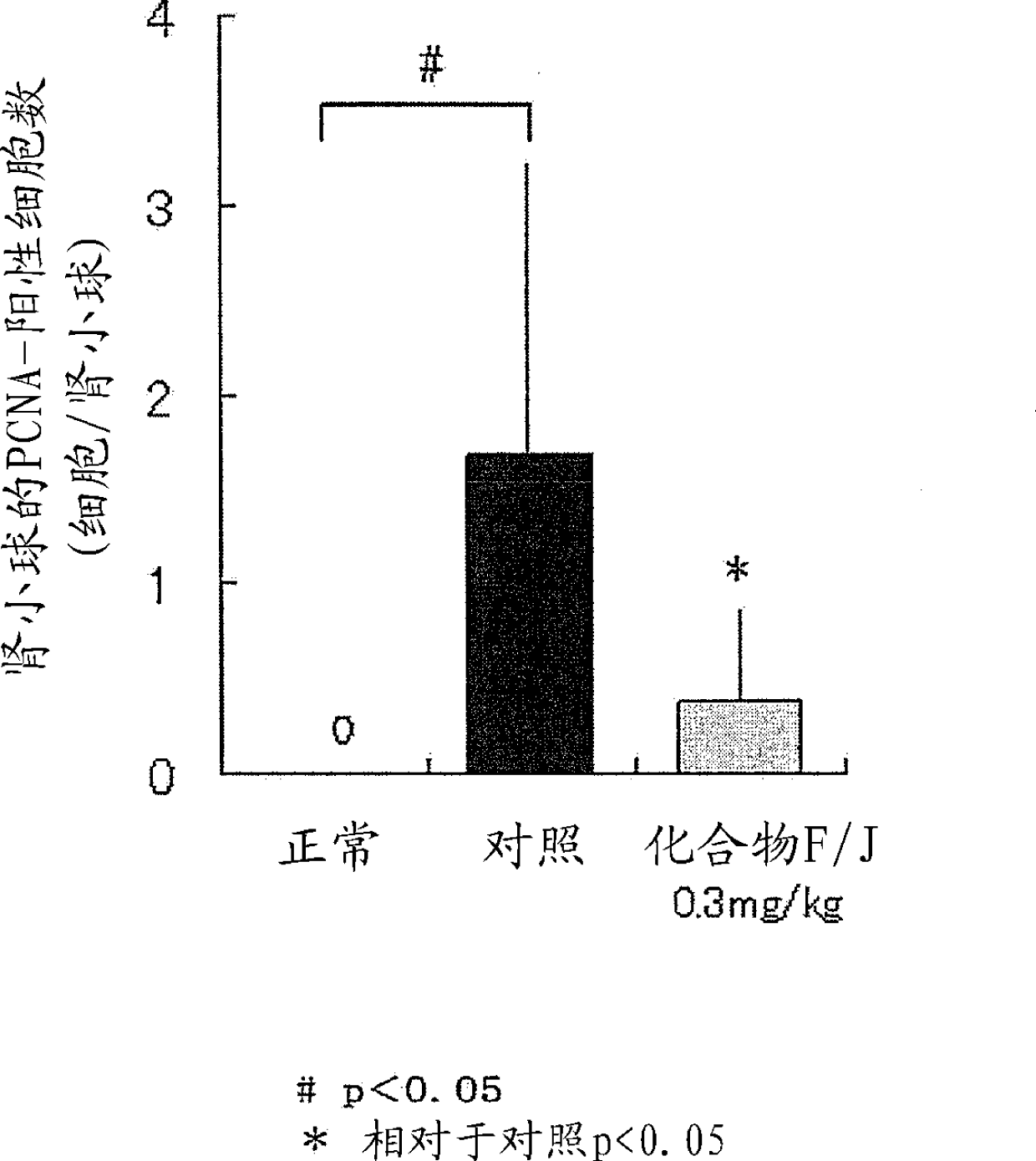
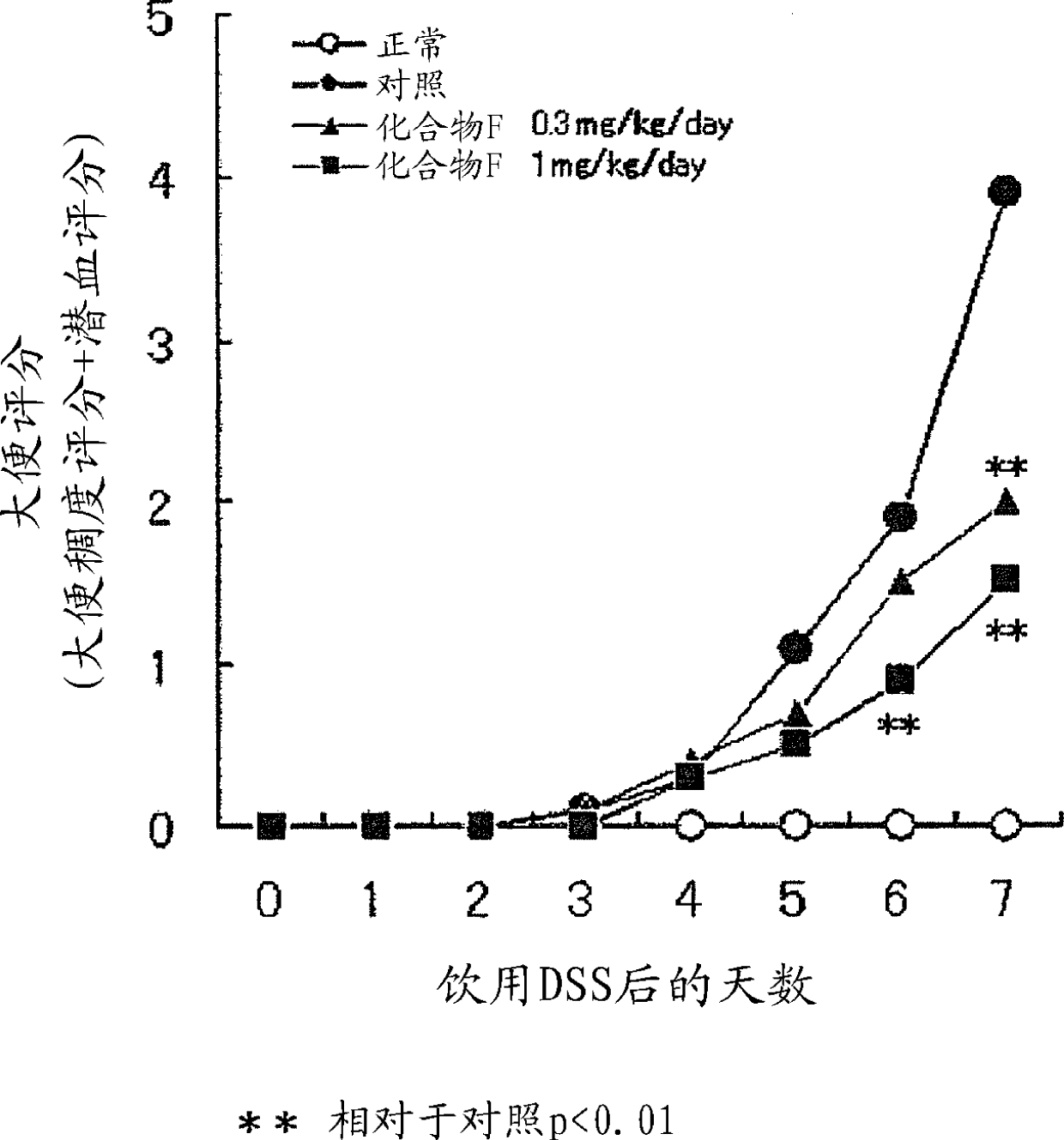
Image
Examples
Embodiment 1
[0238] Synthesis of (2R)-2-(m-tolyl)propionic acid methyl ester
[0239] To (2R)-2-(m-tolyl)propionic acid (12.45 g) were added methanol (14.83 g) and concentrated sulfuric acid (6.46 g), and the mixture was stirred under reflux for 6 hours. Next, the mixture was neutralized with a 10% aqueous sodium carbonate solution, and extracted with hexane. After drying over magnesium sulfate, the residue was concentrated under reduced pressure to obtain the title compound (12.79 g). Its structural properties are described below.
[0240] 1 H-NMR (CDCl 3 ):δ 1.49(d, J=7.0 Hz, 3H), 2.33(s, 3H), 3.64(s, 3H), 3.69(dd, J=14.4, 7.3 Hz, 1H), 7.06-7.22(m, 4H ).
Embodiment 2
[0242] Synthesis of (3R)-2-oxo-3-(m-tolyl)butyl phosphonic acid dimethyl ester
[0243] To dimethyl methylphosphonate (1.97 g) was added tetrahydrofuran (THF) (25 ml), and the mixture was cooled to 78°C. n-Butyllithium (1.5M in hexane) (10 ml) was added, and the mixture was stirred for 1 hour. Then, a THF (3.8 ml) solution of the methyl ester {(2R)-2-(m-tolyl)propionic acid methyl ester} (1.34 g) synthesized in Example 1 was added at -78° C., and the mixture was stirred for 2 Hour. The reaction was quenched with 25 mL of saturated aqueous sodium bicarbonate, and the mixture was extracted with ethyl acetate. The extract was dried over magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane / ethyl acetate=5:1 to 1:5) to obtain the title compound (1.63 g). Its structural properties are described below.
[0244] 1 H-NMR (CDCl 3 ):δ 1.39(d, J=6.7 Hz, 3H), 2.34(s, 3H), 2.84(ddd, J=22.3, 14.1, 0.6 Hz, 1H),...
Embodiment 3
[0246] (1S,5R,6R,7R)-6-[(1E,4R)-3-oxo-4-(m-tolyl)-1-pentenyl]-7-benzoyloxy-2-oxo Synthesis of Heterobicyclo[3.3.0]octan-3-one
[0247] Sodium hydride (55%) (8.75 g) was dispersed in 1,2-dimethoxyethane (DME) (300 ml), and the mixture was ice-cooled. A DME (50 ml) solution of phosphonate {(3R)-2-oxo-3-(m-tolyl)butyl phosphonic acid dimethyl ester} (54.7 g) synthesized in Example 2 was added, and the mixture was stirred for 1 Hour. To the above solution was added (1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one (50.0 g) in DME (400ml) solution and the mixture was stirred for 1 hour. The reaction was quenched with 350 ml of 10% brine, and the mixture was extracted with ethyl acetate. The extract was dried over magnesium sulfate, and the residue was concentrated under reduced pressure. The concentrated crude product was recrystallized from tert-butyl methyl ether to obtain the title compound (64.7 g). Its structural properties are described below.
[0248]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com