Injection of piperacillin-sulbactum sodium medicine composition and preparation method thereof
A technology of piperacillin and sulbactam sodium and sulbactam sodium, which is applied in the field of injection formulations and its preparation, can solve the problems of unstable solutions of piperacillin and sulbactam sodium preparations, unsatisfactory dissolution speed, Can not meet the quality requirements and other problems, to achieve the effect of rapid dissolution, reduce static electricity, and small fluctuation range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Screening of Piperacillin-Sulbactam Sodium Pharmaceutical Composition Injection Formulation
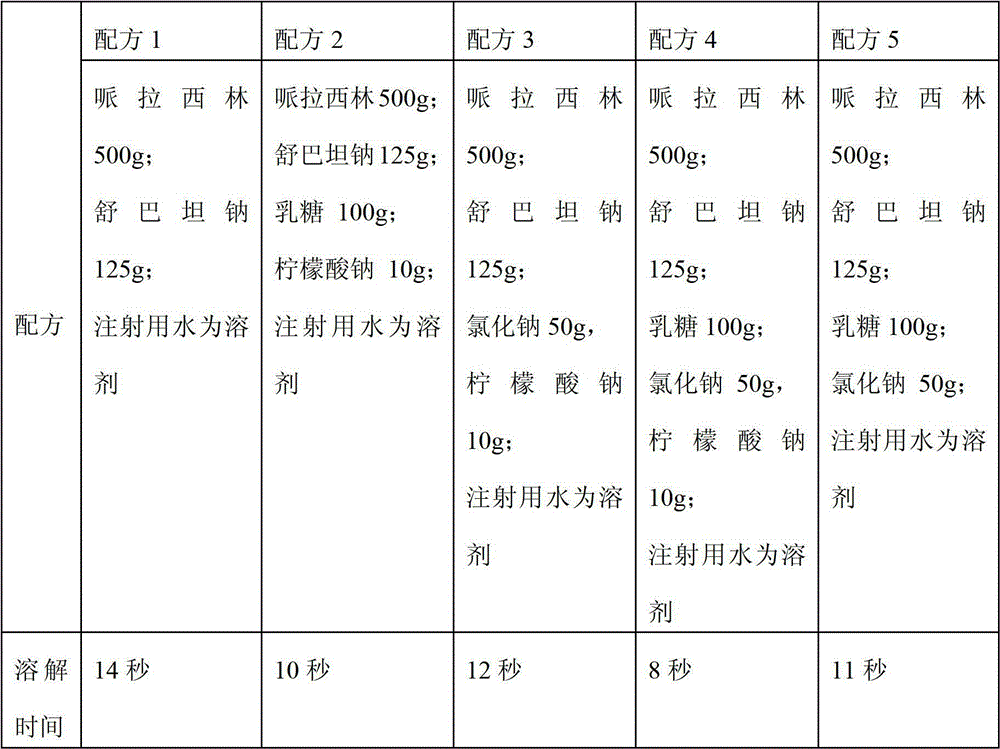
[0030] The pharmaceutical composition injection of the present invention comprises piperacillin and sulbactam sodium, respectively adding one or more of lactose, sodium chloride or sodium citrate, and taking the dissolution time as an index to carry out the pharmaceutical combination of the present invention Table 1 shows the results of the screening experiment of the drug injection formulation.
[0031] Table 1 Formula screening experiment based on dissolution time as index
[0032]
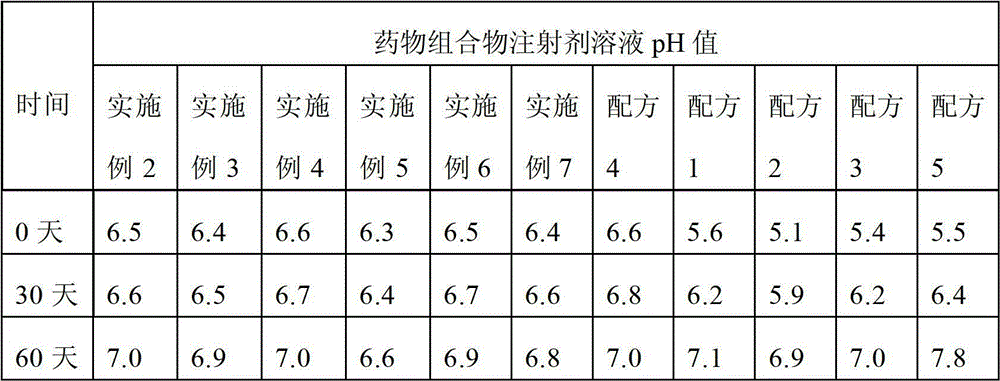
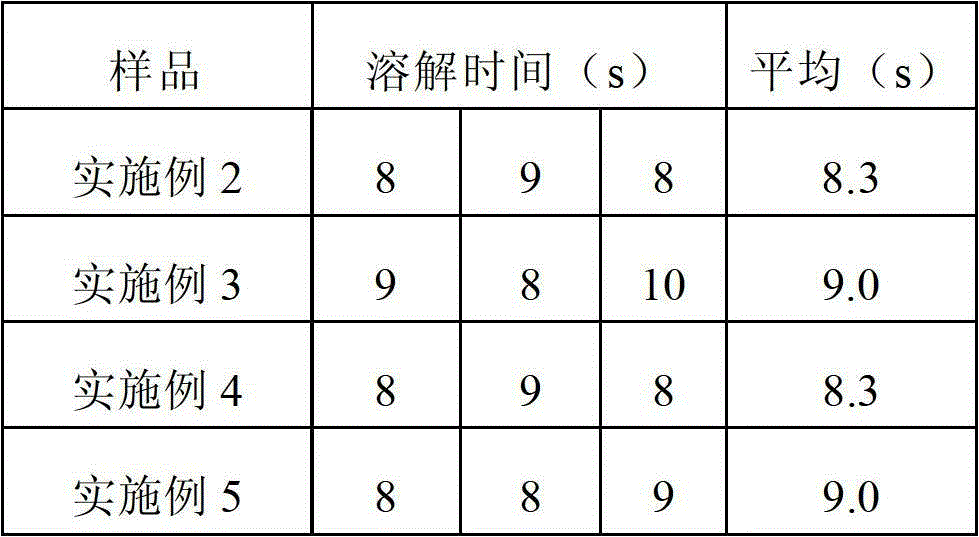
[0033] The experimental results in Table 1 show that when the dissolution time is used as an index, formula 4 is optimal, that is, under the condition that the content of each component added in the formula is the same, piperacillin, sulbactam sodium, lactose, sodium chloride, The formulation consisting of sodium citrate required the shortest dissolution time, taking only 8 seconds...
Embodiment 2
[0034] Example 2 Piperacillin-sulbactam sodium pharmaceutical composition injection and its preparation
[0035] The prescription of pharmaceutical composition injection (1000):
[0036] Piperacillin 250g;
[0037] Sulbactam sodium 62.5g;
[0038] Lactose 50g;
[0039] Sodium chloride 25g;
[0040] Sodium citrate 2.5g;
[0041] Preparation:
[0042] Take the above-mentioned amount of piperacillin, sulbactam sodium, lactose, and sodium chloride, dissolve them in water for injection, make sodium citrate into a 0.1mol / L solution with water for injection, and drop them into the above-mentioned solution after the pH value is 6.8 Stop dripping, then dilute to 5000mL with water for injection, filter through a filter membrane, the pore size of the filter membrane is preferably 0.22um, spray dry, aseptically pulverize, fill, and obtain the piperacillin sulbactam sodium pharmaceutical composition injection.
Embodiment 3
[0043] Example 3 Piperacillin-sulbactam sodium pharmaceutical composition injection and its preparation
[0044] The prescription of pharmaceutical composition injection (1000):
[0045] Piperacillin 2500g;
[0046] Sulbactam sodium 625g;
[0047] Lactose 1000g;
[0048] Sodium chloride 250g;
[0049] Sodium citrate 25g;
[0050] Preparation:
[0051] Take the above-mentioned amount of piperacillin, sulbactam sodium, lactose, and sodium chloride, dissolve them in water for injection, make sodium citrate into a 0.1mol / L solution with water for injection, and drop them into the above-mentioned solution after the pH value is 6.8 Stop dripping, then dilute to 5000mL with water for injection, filter through a filter membrane, the pore size of the filter membrane is preferably 0.22um, spray dry, aseptically pulverize, fill, and obtain the piperacillin sulbactam sodium pharmaceutical composition injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com