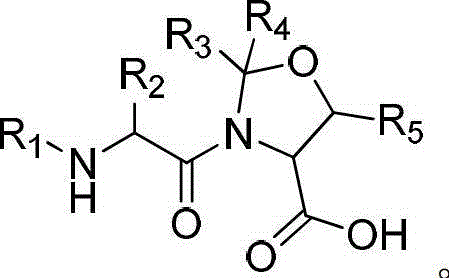
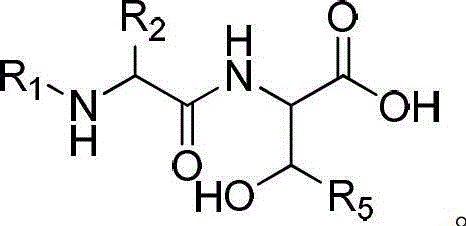
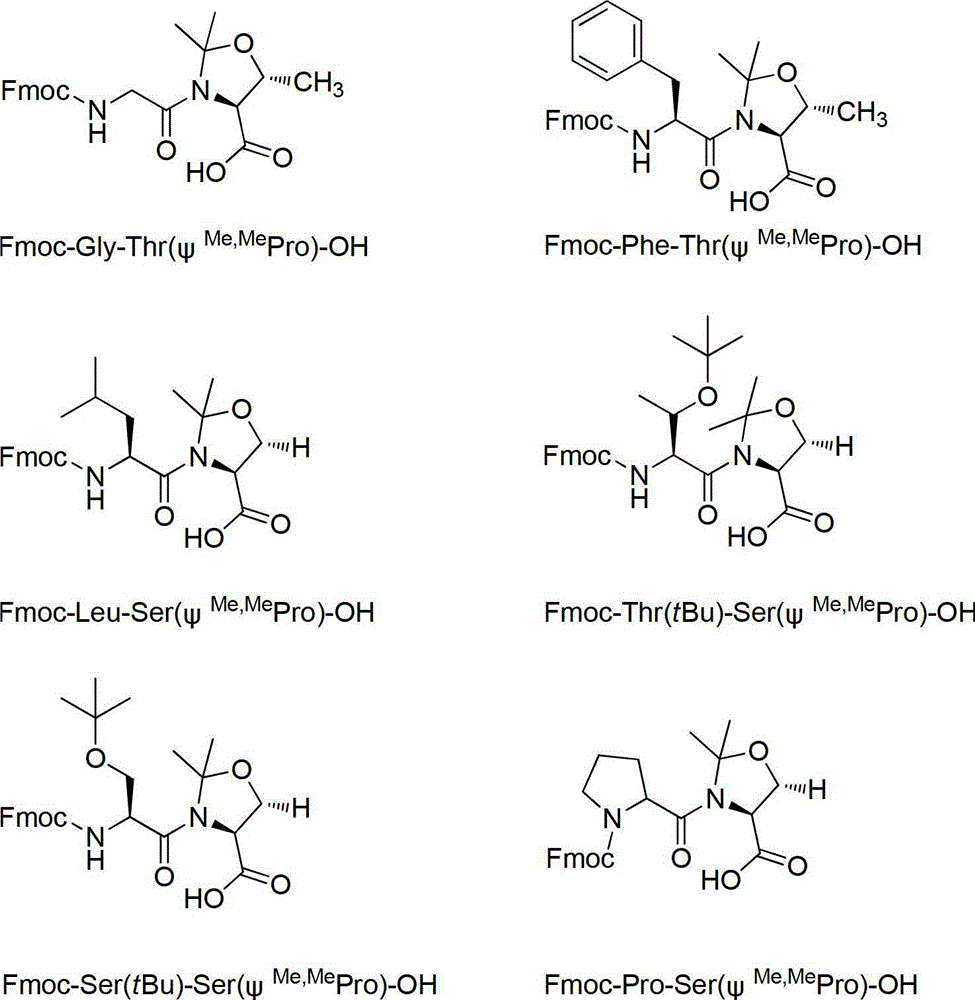
Solid-phase synthesis method of exenatide
A technology of solid-phase synthesis and exenatide, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve problems such as condensation difficulties, fragment dissolution difficulties, and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 prepares pseudoproline Fmoc-Leu-Ser (ψ Me,Me Pro)-OH
[0085] (1) Preparation of Fmoc-Leu-Ser(ψ Me,Me Pro)-OH
[0086] 1) Preparation of Fmoc-Leu-Ser-OBzl
[0087] Add Fmoc-Leu-OH (3.54g, 10mmol), H-Ser-OBzl·HCl (2.32g, 10mmol), HOBt (1.42g, 10.5mmol) into the flask, add DMF (100mL) under ice-cooling, and wait until the solid After dissolving, add DIPEA (3.8mL, 22mmol) to activate for 5min, finally add HBTU (3.98g, 10.5mmol), react for 10min, remove the ice bath, and react at room temperature until the reaction is complete as detected by TLC. Then extracted with ethyl acetate, the organic phase was washed with 1N HCl, saturated NaHCO 3 , washed with saturated NaCl, anhydrous Na 2 SO 4 After drying, the solvent was spin-dried to obtain 5.5 g of white solid, namely Fmoc-Leu-Ser-OBzl.
[0088] 2) Preparation of Fmoc-Leu-Ser(ψ Me,Me Pro)-OBzl
[0089] Add Fmoc-Leu-Ser-OBzl (3.2g, 5mmol), DMP (3.07mL, 25mmol), PPTS (0.377g, 1.5mmol), toluene (100mL) in...
Embodiment 2
[0093] Embodiment 2 prepares the amino acid of Hmb, Dmb or Tmb protection
[0094] (1) Preparation of Fmoc-(Dmb)Gly-OH
[0095] 1) Preparation of H-(Dmb)Gly-OH
[0096] First, the amino acid Gly (7.5g, 100mmol) was dissolved in an aqueous solution of KOH (5.6g, 100mmol), and an ethanol solution of 2,4-dimethoxybenzaldehyde (16.6g, 100mmol) was added to the aqueous solution, and at room temperature After stirring for 30min, slowly add NaBH 4 (4.1g, 101mmol) in aqueous solution (containing a small amount of 1M NaOH). Stir until reaction is complete. Adjust the pH to 4 with concentrated hydrochloric acid, and a solid precipitates out. Wash with ice water and 50% methanol / water solution to obtain 14 g of compound H-(Dmb)Gly-OH with a yield of 62.2%.
[0097] 2) Preparation of Fmoc-(Dmb)Gly-OH
[0098] Dioxane was added to an ice solution of H-(Dmb)Gly-OH (11.25g, 50mmol) until it was completely dissolved, then sodium carbonate (17g, 160mmol) was added, and Fmoc-Cl (27.2g, 10...
Embodiment 3
[0100] Example 3 Preparation of Fmoc-Gly-(Hmb)Gly-OH
[0101] 1) Preparation of H-(Hmb)Gly-OH
[0102] First, the amino acid Gly (7.5g, 100mmol) was dissolved in an aqueous solution of KOH (5.6g, 100mmol), and an ethanol solution of 2,4-dimethoxybenzaldehyde (15.2g, 100mmol) was added to the aqueous solution, and at room temperature After stirring for 30min, slowly add NaBH 4 (4.1g, 101mmol) in aqueous solution (containing a small amount of 1M NaOH). Stir until reaction is complete. Adjust the pH to 4 with concentrated hydrochloric acid, and a solid precipitates out. Wash with ice water and 50% methanol / water solution to obtain 13.8 g of compound H-(Hmb)Gly-OH with a yield of 65.4%.
[0103] 2) Preparation of Fmoc-Gly-OSu
[0104] Fmoc-Gly-OH (2.97g, 10mmol) and N-hydroxysuccinimide (1.15g, 10mmol) were dissolved in DMF, and EDC·HCl (2.30g, 10mmol) was added after cooling in an ice-water bath. The reaction was carried out at room temperature for 24 h, and the reaction wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com